Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0U1IR
|
|||
| Former ID |
DIB014488
|
|||
| Drug Name |
AP-5346
|
|||
| Synonyms |
ProLindac; AP-5286; DACH polymer platinate, Access; DACH-Pt; DACH-Pt prodrug
Click to Show/Hide
|
|||
| Indication | Head and neck cancer [ICD-11: 2D42] | Discontinued in Phase 2 | [1] | |
| Company |
Access Pharmaceuticals Inc
|
|||
| Structure |
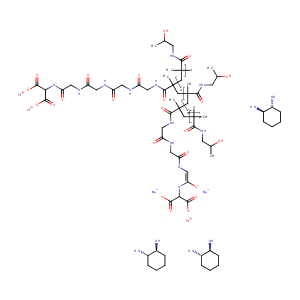 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017581) | |||
| REF 2 | Preclinical efficacy and pharmacokinetics of AP5346, a novel diaminocyclohexane-platinum tumor-targeting drug delivery system. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2248-54. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

