Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0UV1S
|
|||
| Former ID |
DNC013983
|
|||
| Drug Name |
O-methyldauricine
|
|||
| Synonyms |
O-Methyldauricine; 2202-17-7; O,O-Dimethylcuspidaline; O,O-Dimethyldauricinoline; CHEMBL501861; Dauricine, O-methyl- (6CI,7CI,8CI); Isoquinoline, 1,2,3,4-tetrahydro-6,7-dimethoxy-1-((4-(2-methoxy-5-((1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-isoquinolinyl)methyl)phenoxy)phenyl)methyl)-2-methyl-, (R-(R*,R*))-; Dauricine, O-methyl-; AC1L44JA; DTXSID50176486; C39H46N2O6; 8192AH; BDBM50292469; ZINC42805235; LS-85912; AN-50531; 202M177; Q-100278
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
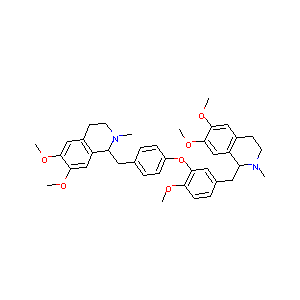 |
Download2D MOL |
||
| Formula |
C39H46N2O6
|
|||
| Canonical SMILES |
CN1CCC2=CC(=C(C=C2C1CC3=CC=C(C=C3)OC4=C(C=CC(=C4)CC5C6=CC(=C(C=C6CCN5C)OC)OC)OC)OC)OC
|
|||
| InChI |
1S/C39H46N2O6/c1-40-16-14-27-21-35(43-4)37(45-6)23-30(27)32(40)18-25-8-11-29(12-9-25)47-39-20-26(10-13-34(39)42-3)19-33-31-24-38(46-7)36(44-5)22-28(31)15-17-41(33)2/h8-13,20-24,32-33H,14-19H2,1-7H3/t32-,33-/m1/s1
|
|||
| InChIKey |
UHYCXSGUNAWVBW-CZNDPXEESA-N
|
|||
| CAS Number |
CAS 2202-17-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dopamine transporter (DAT) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Dopaminergic synapse | |||
| Parkinson's disease | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| Parkinson disease | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| Reactome | Na+/Cl- dependent neurotransmitter transporters | |||
| WikiPathways | Monoamine Transport | |||
| NRF2 pathway | ||||
| Dopaminergic Neurogenesis | ||||
| Parkinsons Disease Pathway | ||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | ||||
| Neurotransmitter Clearance In The Synaptic Cleft | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Effects of various isoquinoline alkaloids on in vitro 3H-dopamine uptake by rat striatal synaptosomes. J Nat Prod. 1995 Oct;58(10):1475-84. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

