Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V0SL
|
|||
| Former ID |
DNCL002505
|
|||
| Drug Name |
Abexinostat
|
|||
| Synonyms |
PCI-24781; 783355-60-2; ABEXINOSTAT; PCI 24781; CRA-024781; CRA 024781; UNII-IYO470654U; 3-((dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)benzofuran-2-carboxamide; CRA-02478; Abexinostat(PCI-24781); PCI-24781 (Abexinostat); Abexinostat (PCI-24781); IYO470654U; 3-[(Dimethylamino)methyl]-N-[2-[4-[(hydroxyamino)carbonyl]phenoxy]ethyl]-2-benzofurancarboxamide; 3-((Dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)-phenoxy)ethyl)benzofuran-2-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Follicular lymphoma [ICD-11: 2A80; ICD-9: 202] | Phase 3 | [1] | |
| Renal cell carcinoma [ICD-11: 2C90; ICD-10: C64; ICD-9: 189] | Phase 3 | [2] | ||
| Acute myeloid leukaemia [ICD-11: 2A60] | Phase 2 | [1] | ||
| Breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [1] | ||
| Diffuse large B-cell lymphoma [ICD-11: 2A81; ICD-10: C83.3; ICD-9: 200] | Phase 2 | [1] | ||
| Gynecologic cancer [ICD-11: 2F33-2F76] | Phase 2 | [1] | ||
| Peripheral T-cell lymphoma [ICD-11: 2A90.C; ICD-10: C84.4; ICD-9: 202.7] | Phase 2 | [1] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2 | [1] | ||
| Company |
Pharmacyclics
|
|||
| Structure |
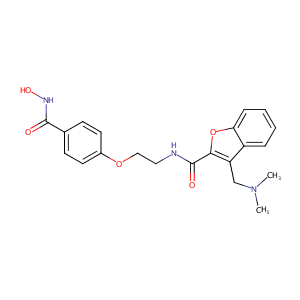 |
Download2D MOL |
||
| Formula |
C21H23N3O5
|
|||
| Canonical SMILES |
CN(C)CC1=C(OC2=CC=CC=C21)C(=O)NCCOC3=CC=C(C=C3)C(=O)NO
|
|||
| InChI |
1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25)
|
|||
| InChIKey |
MAUCONCHVWBMHK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 783355-60-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16857486, 23832872, 42791900, 56269573, 80326339, 117695997, 124756990, 125163795, 131480753, 134223063, 134964420, 135267137, 135626780, 136340216, 136367289, 136367841, 136920360, 137216456, 139896178, 144116341, 152258290, 152344189, 160647129, 160681721, 162011829, 162037426, 162202783, 163907942, 164041979, 164193977, 172914354, 174006344, 174530736, 175426351, 177748391, 186014504, 198941643, 201507997, 223381303, 223705150, 224769568, 226766445, 248596262, 252166578, 252215998, 252220173, 252671930
|
|||
| ChEBI ID |
CHEBI:92223
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histone deacetylase (HDAC) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | ClinicalTrials.gov (NCT03592472) A Study of Pazopanib With or Without Abexinostat in Patients With Locally Advanced or Metastatic Renal Cell Carcinoma (RENAVIV). U.S. National Institutes of Health. | |||
| REF 3 | Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Invest New Drugs. 2015 Apr;33(2):423-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

