Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V3HP
|
|||
| Former ID |
DIB006346
|
|||
| Drug Name |
MP-0112
|
|||
| Synonyms |
DARPin (age related macular degeneration), Molecular Partners/Allergan; VEGF-A specific DARPin, Molecular Partners/Allergan
Click to Show/Hide
|
|||
| Indication | Macular degeneration [ICD-11: 9B78.3; ICD-10: H35.3; ICD-9: 362.5] | Phase 1 | [1] | |
| Company |
Molecular Partners AG
|
|||
| Structure |
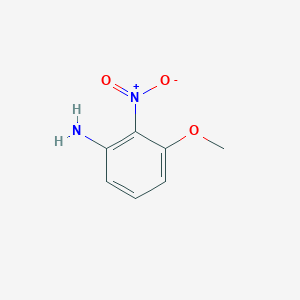 |
Download2D MOL |
||
| Formula |
C7H8N2O3
|
|||
| Canonical SMILES |
COC1=CC=CC(=C1[N+](=O)[O-])N
|
|||
| InChI |
1S/C7H8N2O3/c1-12-6-4-2-3-5(8)7(6)9(10)11/h2-4H,8H2,1H3
|
|||
| InChIKey |
RITQAMSEQYWFML-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 16554-47-5
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01042678) Study of MP0112 Intravitreal Injection in Patients With Diabetic Macular Edema. U.S. National Institutes of Health. | |||
| REF 2 | Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2014 Oct;158(4):724-732.e2. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

