Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V4ZN
|
|||
| Former ID |
DNCL002984
|
|||
| Drug Name |
Cobicistat/darunavir
|
|||
| Synonyms |
Prezcobix; Cobicistat / darunavir; Darunavir / Cobicistat; Cobicistat and darunavir; Cobicistat mixture with darunavir; Darunavir and cobicistat
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Approved | [1] | |
| Company |
Gilead Sciences
|
|||
| Structure |
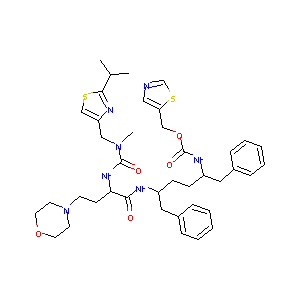 |
Download2D MOL |
||
| Formula |
C67H90N10O12S3
|
|||
| Canonical SMILES |
CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2COC3C2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N.CC(C)C1=NC(=CS1)CN(C)C(=O)NC(CCN2CCOCC2)C(=O)NC(CCC(CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)CC5=CC=CC=C5
|
|||
| InChI |
1S/C40H53N7O5S2.C27H37N3O7S/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35;1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49);3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t32-,33-,36+;22-,23-,24+,25-,26+/m10/s1
|
|||
| InChIKey |
CXORHDVSJFELCV-HWDLLDTBSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cytochrome P450 3A (CYP3A) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014 Feb;74(2):195-206. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

