Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V5XL
|
|||
| Former ID |
DIB005608
|
|||
| Drug Name |
KRI-1314
|
|||
| Synonyms |
CHEMBL3350322; Kri-1314; CHEMBL91826; 4-Cyclohexyl-2-hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4-yl-2-naphthalen-1-ylmethyl-4-oxo-butyrylamino)-propionylamino]-butyric acid isopropyl ester; BDBM50012951; (2R,3S)-3-[N-[(2R)-3-(Morpholinocarbonyl)-2-[(naphthalen-1-yl)methyl]propionyl]-L-histidyl]amino-4-cyclohexyl-2-hydroxybutanoic acid isopropyl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Terminated | [1] | |
| Company |
Kissei Pharmaceutical Co Ltd
|
|||
| Structure |
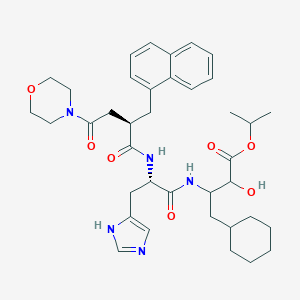 |
Download2D MOL
|
||
| Formula |
C38H51N5O7
|
|||
| Canonical SMILES |
CC(C)OC(=O)C(C(CC1CCCCC1)NC(=O)C(CC2=CN=CN2)NC(=O)C(CC3=CC=CC4=CC=CC=C43)CC(=O)N5CCOCC5)O
|
|||
| InChI |
1S/C38H51N5O7/c1-25(2)50-38(48)35(45)32(19-26-9-4-3-5-10-26)41-37(47)33(22-30-23-39-24-40-30)42-36(46)29(21-34(44)43-15-17-49-18-16-43)20-28-13-8-12-27-11-6-7-14-31(27)28/h6-8,11-14,23-26,29,32-33,35,45H,3-5,9-10,15-22H2,1-2H3,(H,39,40)(H,41,47)(H,42,46)/t29-,32?,33+,35?/m1/s1
|
|||
| InChIKey |
NQXSBCTYTMQGGJ-GBDGZKRDSA-N
|
|||
| CAS Number |
CAS 128053-53-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Angiotensinogenase renin (REN) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Renin-angiotensin system | |||
| Pathwhiz Pathway | Angiotensin Metabolism | |||
| Reactome | Metabolism of Angiotensinogen to Angiotensins | |||
| WikiPathways | ACE Inhibitor Pathway | |||
| Metabolism of Angiotensinogen to Angiotensins | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002396) | |||
| REF 2 | KRI-1314: an orally effective inhibitor of human renin. Jpn J Pharmacol. 1993 Sep;63(1):109-19. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

