Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0VE1W
|
|||
| Former ID |
DPR000152
|
|||
| Drug Name |
SB-623
|
|||
| Synonyms |
(E)-3-[1-(2-diethylaminoethyl)-2-phenethylbenzimidazol-5-yl]-N-hydroxyprop-2-enamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cerebrovascular ischaemia [ICD-11: 8B1Z; ICD-10: I67.8; ICD-9: 434.91] | Phase 2 | [1] | |
| Ischemic stroke [ICD-11: 8B11.5Z; ICD-9: 434.91] | Phase 2 | [2] | ||
| Stroke [ICD-11: 8B20; ICD-10: I64] | Phase 2 | [3] | ||
| Traumatic brain injury [ICD-11: NA07.Z] | Phase 2 | [3] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
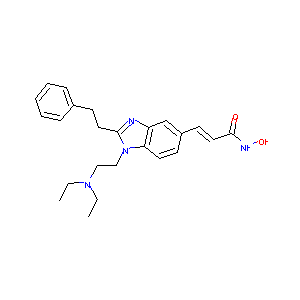 |
Download2D MOL |
||
| Formula |
C24H30N4O2
|
|||
| Canonical SMILES |
CCN(CC)CCN1C2=C(C=C(C=C2)C=CC(=O)NO)N=C1CCC3=CC=CC=C3
|
|||
| InChI |
1S/C24H30N4O2/c1-3-27(4-2)16-17-28-22-13-10-20(12-15-24(29)26-30)18-21(22)25-23(28)14-11-19-8-6-5-7-9-19/h5-10,12-13,15,18,30H,3-4,11,14,16-17H2,1-2H3,(H,26,29)/b15-12+
|
|||
| InChIKey |
FFXUDLUXUIJFSS-NTCAYCPXSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02448641) Study of Modified Stem Cells (SB623) in Patients With Chronic Motor Deficit From Ischemic Stroke. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Histone deacetylase inhibitors in cancer therapy: latest developments, trends and medicinal chemistry perspective. Anticancer Agents Med Chem. 2007 Sep;7(5):576-92. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

