Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0VR7W
|
|||
| Former ID |
DAP000851
|
|||
| Drug Name |
Gemifloxacin
|
|||
| Synonyms |
Factiv; Factive; Gemifloxacin [INN]; Gemifloxacin mesilate; LB 20304; LB 20304a; SB 265805; Factiv (TN); Factive (TN); Gemifloxacin (INN); LB-20304; SB-265805; 7-(3-Aminomethyl)-4-methoxyimino-pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; 7-(3-Aminomethyl-4-methoxyimino-pyrrolidine-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-(1,8)-naphthyridine-3-carboxylic acid; 7-[3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bacterial infection [ICD-11: 1A00-1C4Z; ICD-10: A00-B99] | Approved | [1] | |
| Therapeutic Class |
Antibiotics
|
|||
| Company |
Oscient Pharmaceuticals
|
|||
| Structure |
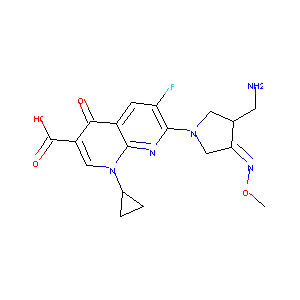 |
Download2D MOL |
||
| Formula |
C18H20FN5O4
|
|||
| Canonical SMILES |
CON=C1CN(CC1CN)C2=C(C=C3C(=O)C(=CN(C3=N2)C4CC4)C(=O)O)F
|
|||
| InChI |
1S/C18H20FN5O4/c1-28-22-14-8-23(6-9(14)5-20)17-13(19)4-11-15(25)12(18(26)27)7-24(10-2-3-10)16(11)21-17/h4,7,9-10H,2-3,5-6,8,20H2,1H3,(H,26,27)/b22-14+
|
|||
| InChIKey |
ZRCVYEYHRGVLOC-HYARGMPZSA-N
|
|||
| CAS Number |
CAS 175463-14-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
610685, 14878468, 23996711, 44709303, 46507905, 48395332, 50070532, 50071315, 50123382, 57372939, 79751939, 85305864, 85789646, 91613407, 96024705, 103165376, 104064190, 124766444, 126665045, 131302500, 134338130, 135029930, 137267488, 141952322, 152047100, 162263733, 163384560, 172861476, 175438039, 179150073, 223664023, 226504227, 238411063, 251890177, 251963973, 252358754
|
|||
| ChEBI ID |
CHEBI:101853
|
|||
| ADReCS Drug ID | BADD_D01013 ; BADD_D01014 | |||
| SuperDrug ATC ID |
J01MA15
|
|||
| SuperDrug CAS ID |
cas=175463146
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacillales | ||||
|
Studied Microbe: Bacillus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bacillus was not significantly changed by Gemifloxacin. | |||
|
Studied Microbe: Staphylococcus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Staphylococcus was not significantly changed by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bacteroides was not significantly changed by Gemifloxacin. | |||
|
Studied Microbe: Bacteroides fragilis
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bacteroides fragilis was decreased by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bifidobacteriales | ||||
|
Studied Microbe: Bifidobacterium
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bifidobacterium was not significantly changed by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Enterobacterales | ||||
|
Studied Microbe: Enterobacteriaceae
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Enterobacteriaceae was decreased by Gemifloxacin. | |||
|
Studied Microbe: Escherichia
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Escherichia was decreased by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Clostridium
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Clostridium was not significantly changed by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Enterococcus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Enterococcus was decreased by Gemifloxacin. | |||
|
Studied Microbe: Streptococcus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Streptococcus was decreased by Gemifloxacin. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Veillonellales | ||||
|
Studied Microbe: Veillonella
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Veillonella was not significantly changed by Gemifloxacin. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial DNA gyrase (Bact gyrase) | Target Info | Modulator | [4] |
| Staphylococcus Topoisomerase IV (Stap-coc parC) | Target Info | Modulator | [4] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Emerging drugs for bacterial urinary tract infections. Expert Opin Emerg Drugs. 2005 May;10(2):275-98. | |||
| REF 2 | Impact of gemifloxacin on the normal human intestinal microflora. J Chemother. 2001 Feb;13(1):47-51. | |||
| REF 3 | Effects of single oral doses of gemifloxacin (320 milligrams) versus trovafloxacin (200 milligrams) on fecal flora in healthy volunteers. Antimicrob Agents Chemother. 2001 Feb;45(2):608-11. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

