Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0W1US
|
|||
| Former ID |
DIB002003
|
|||
| Drug Name |
PLECONARIL
|
|||
| Synonyms |
Picovir; Pleconaril < Prop INN; SR-263843; SR-63843; VP-63843; Win-63843; 3-[3,5-Dimethyl-4-[3-(3-methylisoxazol-5-yl)propoxy]phenyl]-5-(trifluoromethyl)-1,2,4-oxadiazole
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Virus infection [ICD-11: 1A24-1D9Z] | Phase 2 | [1] | |
| Company |
ViroPharma
|
|||
| Structure |
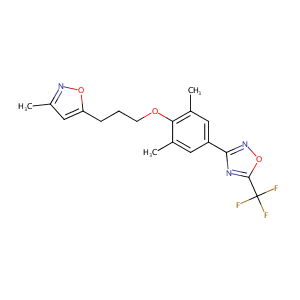 |
Download2D MOL |
||
| Formula |
C18H18F3N3O3
|
|||
| Canonical SMILES |
CC1=CC(=CC(=C1OCCCC2=CC(=NO2)C)C)C3=NOC(=N3)C(F)(F)F
|
|||
| InChI |
1S/C18H18F3N3O3/c1-10-7-13(16-22-17(27-24-16)18(19,20)21)8-11(2)15(10)25-6-4-5-14-9-12(3)23-26-14/h7-9H,4-6H2,1-3H3
|
|||
| InChIKey |
KQOXLKOJHVFTRN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 153168-05-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Picornavirus Capsid protein VP1 (PicVir VP1) | Target Info | Modulator | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00031512) Pleconaril Enteroviral Sepsis Syndrome. U.S. National Institutes of Health. | |||
| REF 2 | Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother. 2005 Nov;49(11):4492-9. | |||
| REF 3 | Treatment of picornavirus infections. Antiviral Res. 2002 Feb;53(2):83-98. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

