Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0WF7L
|
|||
| Former ID |
DCL000499
|
|||
| Drug Name |
Brilinta
|
|||
| Synonyms |
Ticagrelor; 274693-27-5; Brilique; AZD-6140; AZD6140; AZD 6140; AR-C126532XX; UNII-GLH0314RVC; [14C]-Ticagrelor; GLH0314RVC; CHEBI:68558; (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-DIFLUOROPHENYL)CYCLOPROPYL]AMINO]-5-(PROPYLTHIO)-3H-1,2,3-TRIAZOLO[4,5-D]PYRIMIDIN-3-YL]-5-(2-HYDROXYETHOXY)-1,2-CYCLOPENTANEDIOL; (1S,2S,3R,5S)-3-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol; Brilique; AR-C126532; Ticagrelor (USAN/INN); Brilinta/Brilique
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Thrombosis [ICD-11: DB61-GB90; ICD-10: I80-I82] | Approved | [1], [2] | |
| Arterial thrombosis [ICD-11: DB61-DD30] | Phase 3 | [3] | ||
| Myocardial infarction [ICD-11: BA41-BA43] | Phase 3 | [3] | ||
| Company |
AstraZeneca
|
|||
| Structure |
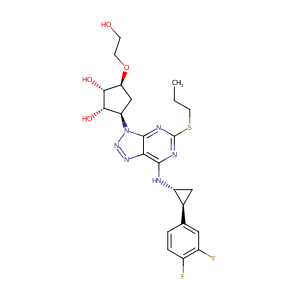 |
Download2D MOL |
||
| Formula |
C23H28F2N6O4S
|
|||
| Canonical SMILES |
CCCSC1=NC(=C2C(=N1)N(N=N2)C3CC(C(C3O)O)OCCO)NC4CC4C5=CC(=C(C=C5)F)F
|
|||
| InChI |
1S/C23H28F2N6O4S/c1-2-7-36-23-27-21(26-15-9-12(15)11-3-4-13(24)14(25)8-11)18-22(28-23)31(30-29-18)16-10-17(35-6-5-32)20(34)19(16)33/h3-4,8,12,15-17,19-20,32-34H,2,5-7,9-10H2,1H3,(H,26,27,28)/t12-,15+,16+,17-,19-,20+/m0/s1
|
|||
| InChIKey |
OEKWJQXRCDYSHL-FNOIDJSQSA-N
|
|||
| CAS Number |
CAS 274693-27-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14836432, 14860737, 24162024, 45293678, 49684205, 57373617, 77877844, 92729712, 96025700, 103554312, 123051091, 124759706, 126644885, 126666051, 135649975, 136345665, 137171656, 139857160, 152040158, 152258075, 160645837, 160646914, 162011636, 163346229, 163383616, 164178089, 164765598, 165245536, 170501033, 171063069, 172914585, 174528632, 175267325, 175427101, 185974765, 189657701, 198993342, 211534943, 223384813, 223666650, 224638556, 228139491, 247988848, 249582784, 249807649, 249807650, 249816423, 251966736, 251970939, 252216468
|
|||
| ChEBI ID |
CHEBI:68558
|
|||
| ADReCS Drug ID | BADD_D02214 | |||
| SuperDrug ATC ID |
B01AC24
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | P2Y purinoceptor 12 (P2RY12) | Target Info | Antagonist | [4], [5] |
| KEGG Pathway | Platelet activation | |||
| Reactome | P2Y receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Signal amplification | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1765). | |||
| REF 2 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||
| REF 3 | ClinicalTrials.gov (NCT01732822) A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 328). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

