Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0X0CH
|
|||
| Former ID |
DCL000811
|
|||
| Drug Name |
Ganaxolone
|
|||
| Synonyms |
Ganaxolone [USAN]; CCD 1042; CCD-1042; Ganaxolone (USAN/INN); (3alpha,5alpha)-3-Hydroxy-3-methylpregnan-20-one; 1-[(3R,5S,10S,13S,17S)-3-hydroxy-3,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]ethanone; 1-[(3R,5S,8R,9S,10S,13S,14S,17S)-3-hydroxy-3,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]ethanone; 3alpha-Hydroxy-3-methyl-5alpha-pregnan-20-one; 3alpha-Hydroxy-3beta-methyl-5alpha-pregnan-20-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epileptic seizures [ICD-11: 8A61-8A6Z; ICD-9: 345.9, 780.3] | Approved | [1] | |
| Complex partial seizure [ICD-11: 8A68.0; ICD-9: 345.4] | Phase 3 | [2] | ||
| Postpartum depression [ICD-11: 6E20.0] | Phase 2 | [3] | ||
| Company |
Marinus
|
|||
| Structure |
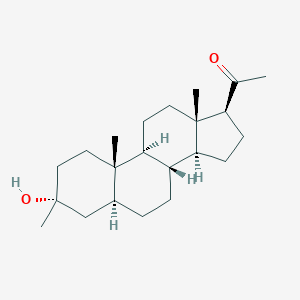 |
Download2D MOL |
||
| Formula |
C22H36O2
|
|||
| Canonical SMILES |
CC(=O)C1CCC2C1(CCC3C2CCC4C3(CCC(C4)(C)O)C)C
|
|||
| InChI |
1S/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16-,17+,18-,19-,20+,21-,22+/m0/s1
|
|||
| InChIKey |
PGTVWKLGGCQMBR-FLBATMFCSA-N
|
|||
| CAS Number |
CAS 38398-32-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | GABA(A) receptor (GABAR) | Target Info | Modulator | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 215904. | |||
| REF 2 | ClinicalTrials.gov (NCT01963208) Phase 3 Study of Adjunctive Ganaxolone in Adults With Drug-resistant Partial Onset Seizures and Open-label Extension. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

