Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0XX9Y
|
|||
| Former ID |
DIB019727
|
|||
| Drug Name |
DH97
|
|||
| Synonyms |
DH 97; DH97; 343263-95-6; N-[2-(2-benzyl-1H-indol-3-yl)ethyl]pentanamide; N-(2-(2-Benzyl-1H-indol-3-yl)ethyl)pentanamide; Luzindole,N-pentanoyl; Tocris-1218; AC1MRF3Z; 220339-00-4; N-pentanoyl 2-benzyltryptamine; N-Pentanoyl-2-benzyltryptamine; SCHEMBL1626901; GTPL3366; CHEMBL1327247; CHEBI:92107; CTK8E7620; BDBM85384; DTXSID90392749; MolPort-003-983-554; HMS3267D11; ZINC2581407; BN0182; AKOS024456472; NCGC00025049-01; NCGC00025049-02; ACM343263956; RT-012298; FT-0763744; SR-01000597373; SR-01000597373-1; J-019556
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
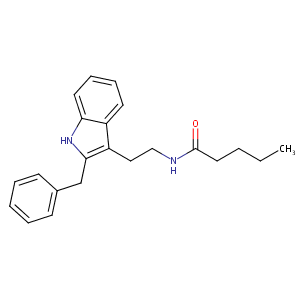 |
Download2D MOL
|
||
| Formula |
C22H26N2O
|
|||
| Canonical SMILES |
CCCCC(=O)NCCC1=C(NC2=CC=CC=C21)CC3=CC=CC=C3
|
|||
| InChI |
1S/C22H26N2O/c1-2-3-13-22(25)23-15-14-19-18-11-7-8-12-20(18)24-21(19)16-17-9-5-4-6-10-17/h4-12,24H,2-3,13-16H2,1H3,(H,23,25)
|
|||
| InChIKey |
HDOIPCLEKCEANF-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:92107
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Melatonin receptor type 1A (MTNR1A) | Target Info | Antagonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Circadian entrainment | ||||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3366). | |||
| REF 2 | Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores. Naunyn Schmiedebergs Arch Pharmacol. 1998 Nov;358(5):522-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

