Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y2LR
|
|||
| Former ID |
DAP000859
|
|||
| Drug Name |
Doxylamine
|
|||
| Synonyms |
Dossilamina; Doxilamina; Doxilminio; Doxylaminum; Dossilamina [DCIT]; Diclectin (TN); Dolased (TN); Donormyl (TN); Dormidina (TN); Doxilminio [INN-Spanish]; Doxylamine (INN); Doxylamine [INN:BAN]; Doxylaminum [INN-Latin]; Dozile (TN); Evanorm (TN); Mersyndol (TN); Restavit (TN); Somnil (TN); Syndol (TN); Unisom-2 (TN); Phenyl-2-pyridylmethyl-beta-N,N-dimethylaminoethyl ether; N,N-Dimethyl-2-(1-phenyl-1-(2-pyridinyl)ethoxy)ethanamine; N,N-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine; N,N-dimethyl-2-[(1-phenyl-1-pyridin-2-ylethyl)oxy]ethanamine; N,N-dimethyl-2-[1-phenyl-1-(pyridin-2-yl)ethoxy]ethanamine; 2-(alpha-(2-(Dimethylamino)ethoxy)-alpha-methylbenzyl)pyridine; 2-Dimethylaminoethoxyphenylmethyl-2-picoline
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Morning sickness [ICD-11: SC00] | Approved | [1], [2] | |
| Therapeutic Class |
Hypnotics and Sedatives
|
|||
| Company |
Ion Healthcare
|
|||
| Structure |
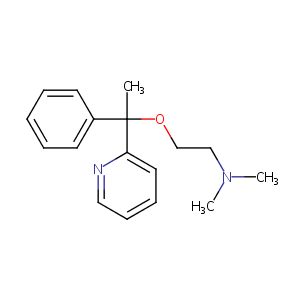 |
Download2D MOL |
||
| Formula |
C17H22N2O
|
|||
| Canonical SMILES |
CC(C1=CC=CC=C1)(C2=CC=CC=N2)OCCN(C)C
|
|||
| InChI |
1S/C17H22N2O/c1-17(20-14-13-19(2)3,15-9-5-4-6-10-15)16-11-7-8-12-18-16/h4-12H,13-14H2,1-3H3
|
|||
| InChIKey |
HCFDWZZGGLSKEP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 469-21-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4407403, 7979131, 8152014, 10523560, 11336016, 11361255, 11363908, 11366470, 11369032, 11371258, 11374274, 11377194, 11462227, 11466055, 11467175, 11483866, 11485750, 11487931, 11490225, 11492343, 11494828, 14872648, 29222304, 46506354, 47736532, 47736533, 47810800, 47885461, 47959795, 48035178, 48185042, 48334546, 48415929, 49698860, 49854351, 50105257, 50105258, 50105259, 52078799, 53787513, 56464430, 85209768, 85788879, 90340822, 92714613, 96024574, 99281835, 103266950, 104302705, 124749641
|
|||
| ChEBI ID |
CHEBI:51380
|
|||
| ADReCS Drug ID | BADD_D02418 | |||
| SuperDrug ATC ID |
R06AA09
|
|||
| SuperDrug CAS ID |
cas=000469216
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [3], [4] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7171). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040167. | |||
| REF 3 | First-generation H1 antihistamines found in pilot fatalities of civil aviation accidents, 1990-2005. Aviat Space Environ Med. 2007 May;78(5):514-22. | |||
| REF 4 | Steady-state brain concentrations of antihistamines in rats: interplay of membrane permeability, P-glycoprotein efflux and plasma protein binding. Pharmacology. 2004 Oct;72(2):92-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

