Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y7YD
|
|||
| Former ID |
DIB006364
|
|||
| Drug Name |
SMT-C1100
|
|||
| Synonyms |
BMN-195; Utrophin modulators (Duchenne muscular dystrophy); VOXC-1100; Utrophin modulators (Duchennemuscular dystrophy), VASTox; Utorphin upregulators (Duchenne muscular dystrophy), VASTox/University of Oxford; Utrophin modulators (Duchenne muscular dystrophy), Summit/BioMarin; Utropine inducer (Duchenne muscular dystrophy), Summit/BioMarin
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Duchenne dystrophy [ICD-11: 8C70; ICD-10: G71.0] | Phase 2 | [1] | |
| Company |
Summit Corp plc
|
|||
| Structure |
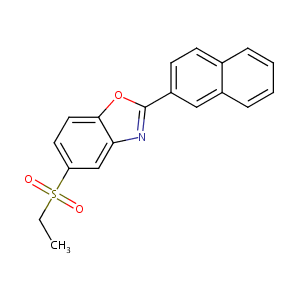 |
Download2D MOL |
||
| Formula |
C19H15NO3S
|
|||
| Canonical SMILES |
CCS(=O)(=O)C1=CC2=C(C=C1)OC(=N2)C3=CC4=CC=CC=C4C=C3
|
|||
| InChI |
1S/C19H15NO3S/c1-2-24(21,22)16-9-10-18-17(12-16)20-19(23-18)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,2H2,1H3
|
|||
| InChIKey |
KSGCNXAZROJSNW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 945531-77-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Utrophin (UTRN) | Target Info | Regulator (upregulator) | [2] |
| WikiPathways | Primary Focal Segmental Glomerulosclerosis FSGS | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to healthy male adult volunteers. J Clin Pharmacol.2015 Jun;55(6):698-707. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

