Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y7ZU
|
|||
| Former ID |
DAP000533
|
|||
| Drug Name |
Dacarbazine
|
|||
| Synonyms |
Biocarbazin; Biocarbazine; DTIC; DTICDome; DTIE; Dacarbazino; Dacarbazinum; Dacatic; Decarbazine; Deticene; Dimethyltriazenoimidazolecarboxamide; ICDMT; ICDT; Biocarbazine R; DTIC Dome; Dimethyl Imidazole Carboxamide; Dimethyl Triazeno Imidazole Carboxamide; Imidazole carboxamide; HE1150000; Carboxamide (TN); Carboxamide, Dimethyl Imidazole; DIC (TN); DTIC (TN); DTIC-Dome; Dacarbazino [INN-Spanish]; Dacarbazinum [INN-Latin]; Imidazole (TN); Imidazole Carboxamide, Dimethyl; NPFAPI-05; DTIC-Dome (TN); Di-me-triazenoimidazolecarboxamide; Di-methyl-triazenoimidazolecarboxamide; Dtic-Dome (TN); DTIC, DTIC-Dome, Dacarbazine; Dacarbazine (JAN/USP/INN); Dacarbazine [USAN:INN:BAN:JAN]; (5E)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (5Z)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (Dimethyltriazeno)imidazolecarboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(3,3-Dimethyl-1-triazeno)imidazole-5-carboxamide; 4-(3,3-Dimethyltriazeno)imidazole-5-carboxamide; 4-(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(Dimethyltriazeno)imidazole-5-c arboxamide; 4-(Dimethyltriazeno)imidazole-5-carboxamide; 4-(or 5)-(3,3-Dimethyl-1-triazeno)imidazole-5(or 4)-carboxamide; 4-[(1E)-3,3-Dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 4-[3,3-dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 5(or 4)-(dimethyltriazeno)imidazol e-4(or 5)-carboxamide; 5(or 4)-(dimethyltriazeno)imidazole-4(or 5)-carboxamide; 5-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)-1H-imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)imidazole-4-carboxamide; 5-(3,3-Dimethyltri azeno)imidazole-4-carboxamide; 5-(3,3-Dimethyltriazeno)-imidazole-4-carbamide; 5-(3,3-Dimethyltriazeno)imidazole-4-carboxamide; 5-(3,3-dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide; 5-(Dimethyltriazeno)-4-imidazolecarboxamide; 5-(Dimethyltriazeno)imidazole-4-carboxamide; 5-(Dimethyltriazeno)imidazole-4-carboximide; 5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; 5-[3,3-Dimethyl-1-triazenyl]imidazole-4-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Approved | [1] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Bayer Pharmaceuticals Corporation
|
|||
| Structure |
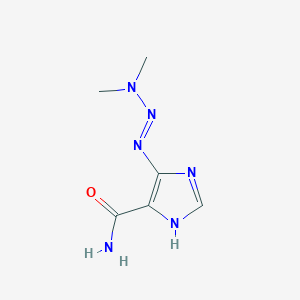 |
Download2D MOL |
||
| Formula |
C6H10N6O
|
|||
| Canonical SMILES |
CN(C)N=NC1=C(NC=N1)C(=O)N
|
|||
| InChI |
1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3H,1-2H3,(H2,7,13)(H,8,9)/b11-10+
|
|||
| InChIKey |
FDKXTQMXEQVLRF-ZHACJKMWSA-N
|
|||
| CAS Number |
CAS 4342-03-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9152, 7847354, 7978483, 10321843, 15147351, 24893683, 39379666, 42680836, 49698562, 49854343, 50263006, 53786765, 57361557, 85789482, 87560054, 92125354, 92309016, 103914325, 113909374, 118816621, 121363025, 121559958, 124455296, 131314849, 134337825, 134985384, 135286428, 135341553, 140170342, 140349396, 143832162, 160964192, 162177330, 164196541, 165298539, 175265531, 175611919, 176484330, 176484580, 176484944, 187072676, 210279185, 210281508, 227267566, 241085945, 251885216, 252401443
|
|||
| ChEBI ID |
CHEBI:94587
|
|||
| ADReCS Drug ID | BADD_D00568 | |||
| SuperDrug ATC ID |
L01AX04
|
|||
| SuperDrug CAS ID |
cas=004342034
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Erysipelotrichales | ||||
|
Studied Microbe: Erysipelatoclostridium ramosum
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Erysipelatoclostridium ramosum was decreased by Dacarbazine (adjusted p-values: 6.96E-04). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Breaker | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 075259. | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Predicting the myelotoxicity of chemotherapy: the use of pretreatment O6-methylguanine-DNA methyltransferase determination in peripheral blood mono... Melanoma Res. 2011 Dec;21(6):502-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

