Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0YG7M
|
|||
| Former ID |
DAP000329
|
|||
| Drug Name |
Ketotifen
|
|||
| Synonyms |
Ketotifene; Ketotifeno; Ketotifenum; Ketotifin; Ketotiphen; Ketotiphene; Zaditor; Ketotifene fumarate; Alaway (TN); HC 20-511; Ketotifen (INN); Ketotifen [INN:BAN]; Ketotifene [INN-French]; Ketotifeno [INN-Spanish]; Ketotifenum [INN-Latin]; Zaditor (TN); 10H-Benzo[4,5]cyclohepta[1,2-b]thiophen-10-one, 4,9-dihydro-4-(1-methyl-4-piperidinylidene)-, (2E)-2-butenedioate (1:1); 4,9-Dihydro-4-(1-methyl-4-piperidinylidene)-10H-benzo(4,5)cyclohepta(1,2-b)thiophen-10-one; 4,9-Dihydro-4-(1-methyl-4-piperidylidene)-10H-benzo(4,5)-cyclohepta(1,2-b)thiophen-10-one; 4-(1-Methyl-4-piperidylidene)-9-hydrobenzo[a]thiopheno[3,2-d][7]annulen-10-one; 4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thia-benzo[f]azulen-10-one; 4-(1-methylpiperidin-4-ylidene)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergic conjunctivitis [ICD-11: 9A60.02; ICD-10: H10.1] | Approved | [1], [2] | |
| Therapeutic Class |
Antiallergic Agents
|
|||
| Company |
Norvatis Phamaceuticals Corporation
|
|||
| Structure |
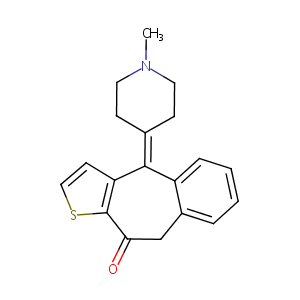 |
Download2D MOL |
||
| Formula |
C19H19NOS
|
|||
| Canonical SMILES |
CN1CCC(=C2C3=C(C(=O)CC4=CC=CC=C42)SC=C3)CC1
|
|||
| InChI |
1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3
|
|||
| InChIKey |
ZCVMWBYGMWKGHF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 34580-13-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
615225, 3937163, 5316935, 7979682, 8152425, 10519499, 11111350, 11111351, 11113338, 11335286, 11360525, 11363320, 11365882, 11368444, 11372283, 11374428, 11376606, 11461497, 11466399, 11467519, 11485011, 11486178, 11489014, 11491197, 11492620, 11494240, 12015272, 14800895, 25672628, 26751502, 29222946, 46508921, 47365031, 47440100, 47588844, 47810604, 47810605, 47959576, 48034958, 48110306, 48259067, 48334334, 48416149, 49698902, 49883766, 50100258, 50104048, 50782134, 53790083, 57322013
|
|||
| ChEBI ID |
CHEBI:92511
|
|||
| ADReCS Drug ID | BADD_D01234 | |||
| SuperDrug ATC ID |
R06AX17; S01GX08
|
|||
| SuperDrug CAS ID |
cas=034580137
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [3], [4] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7206). | |||
| REF 2 | Emerging drugs for ocular allergy. Expert Opin Emerg Drugs. 2005 Aug;10(3):505-20. | |||
| REF 3 | Intact cell binding for in vitro prediction of sedative and non-sedative histamine H1-receptor antagonists based on receptor internalization. J Pharmacol Sci. 2008 May;107(1):66-79. | |||
| REF 4 | Influence of chronic treatment with H1 receptor antagonists on the anticonvulsant activity of antiepileptic drugs. Pol J Pharmacol. 2001 Jan-Feb;53(1):93-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

