Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z4FE
|
|||
| Former ID |
DIB007601
|
|||
| Drug Name |
MIN-117
|
|||
| Indication | Major depressive disorder [ICD-11: 6A70.3; ICD-10: F32.2] | Phase 2 | [1] | |
| Company |
Minerva neurosciences
|
|||
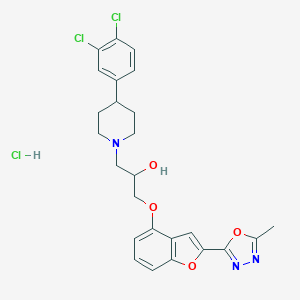
| Structure |
 |
Download2D MOL
|
||
| Formula |
C25H26Cl3N3O4
|
|||
| Canonical SMILES |
CC1=NN=C(O1)C2=CC3=C(O2)C=CC=C3OCC(CN4CCC(CC4)C5=CC(=C(C=C5)Cl)Cl)O.Cl
|
|||
| InChI |
1S/C25H25Cl2N3O4.ClH/c1-15-28-29-25(33-15)24-12-19-22(3-2-4-23(19)34-24)32-14-18(31)13-30-9-7-16(8-10-30)17-5-6-20(26)21(27)11-17;/h2-6,11-12,16,18,31H,7-10,13-14H2,1H3;1H
|
|||
| InChIKey |
HRNDUKHBCUTNAL-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800037483) | |||
| REF 2 | Company report (Minerva Neurosciences),MIN-101,Schizophrenia, 6 trials completed; Once a day formulation completed , Phase IIa completed; Phase IIb enrollment ongoing and expected to continue over the last 3 quarters of 2015. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

