Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z7FU
|
|||
| Former ID |
DIB014033
|
|||
| Drug Name |
INS 316
|
|||
| Synonyms |
Uridine 5'-triphosphate; uridine 5'-triphosphate; uridine triphosphate; 63-39-8; Uteplex; UTP; Uridine 5'-(tetrahydrogen triphosphate); 5'-UTP; Uridine 5'-triphosphoric acid; H4utp; UNII-UT0S826Z60; BRN 0071520; INS316; EINECS 200-558-7; uridine-5'-triphosphate; CHEMBL336296; CHEBI:15713; UT0S826Z60; Uridine5'-(tetrahydrogen triphosphate); [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-phosphoryl] phosphono hydrogen phosphate; uridine5'-triphosphate; uridintriphosphat; uridine tetrahydrogen
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Lung cancer [ICD-11: 2C25.0; ICD-9: 162] | Discontinued in Phase 3 | [1] | |
| Company |
Inspire Pharmaceuticals
|
|||
| Structure |
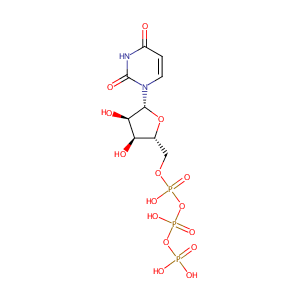 |
Download2D MOL |
||
| Formula |
C9H15N2O15P3
|
|||
| Canonical SMILES |
C1=CN(C(=O)NC1=O)C2C(C(C(O2)COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O)O
|
|||
| InChI |
1S/C9H15N2O15P3/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1
|
|||
| InChIKey |
PGAVKCOVUIYSFO-XVFCMESISA-N
|
|||
| CAS Number |
CAS 63-39-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3375, 584151, 831552, 833275, 833716, 841750, 855457, 7838933, 7891028, 7980869, 8027183, 8027189, 8143371, 8153844, 11335444, 11360683, 11364591, 11367153, 11369715, 11372791, 11374619, 11377877, 11461655, 11484245, 11488341, 11491411, 11492666, 11495511, 14720137, 14761469, 14932630, 17436071, 17436075, 24277146, 24277190, 24775910, 26706259, 26736753, 26737220, 26756678, 29225136, 47365080, 47662170, 47810645, 50061760, 53788170, 56312062, 56312125, 56312759, 56312760
|
|||
| ChEBI ID |
CHEBI:15713
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | P2Y purinoceptor 11 (P2RY11) | Target Info | Agonist | [2] |
| P2Y purinoceptor 2 (P2RY2) | Target Info | Modulator | [3], [4], [5] | |
| P2Y purinoceptor 4 (P2RY4) | Target Info | Agonist | [6] | |
| P2Y purinoceptor 6 (P2RY6) | Target Info | Agonist | [7] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Inflammatory mediator regulation of TRP channels | ||||
| NetPath Pathway | IL5 Signaling Pathway | |||
| Reactome | G alpha (q) signalling events | |||
| P2Y receptors | ||||
| Surfactant metabolism | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008257) | |||
| REF 2 | Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol Pharmacol. 2003 Jun;63(6):1356-63. | |||
| REF 3 | Safety of aerosolized INS 365 in patients with mild to moderate cystic fibrosis: results of a phase I multi-center study. Pediatr Pulmonol. 2001 Aug;32(2):122-8. | |||
| REF 4 | Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors. J Med Chem. 2002 Jan 3;45(1):208-18. | |||
| REF 5 | P2Y(2) receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000 Oct;21(4):782-7. | |||
| REF 6 | ATP, an agonist at the rat P2Y(4) receptor, is an antagonist at the human P2Y(4) receptor. Mol Pharmacol. 2000 May;57(5):926-31. | |||
| REF 7 | Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996 May 15;222(2):303-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

