Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0ZM5W
|
|||
| Former ID |
DIB011483
|
|||
| Drug Name |
Esuprone
|
|||
| Synonyms |
LU-43839; Esuprone; 7-Hydroxy-3,4-dimethyl-2H-1-benzopyran-2-one ethanesulfonate; 7-Hydroxy-3,4-dimethylcoumarin ethanesulfonate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Major depressive disorder [ICD-11: 6A70.3; ICD-10: F32.2] | Discontinued in Phase 2 | [1] | |
| Structure |
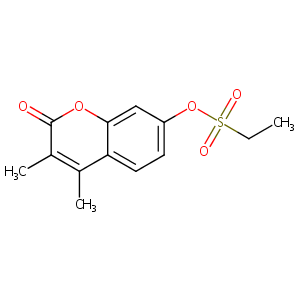 |
Download2D MOL |
||
| Formula |
C13H14O5S
|
|||
| Canonical SMILES |
CCS(=O)(=O)OC1=CC2=C(C=C1)C(=C(C(=O)O2)C)C
|
|||
| InChI |
1S/C13H14O5S/c1-4-19(15,16)18-10-5-6-11-8(2)9(3)13(14)17-12(11)7-10/h5-7H,4H2,1-3H3
|
|||
| InChIKey |
CHDGAVDQRSPBTA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 91406-11-0
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005281) | |||
| REF 2 | MAO-A inhibition in brain after dosing with esuprone, moclobemide and placebo in healthy volunteers: in vivo studies with positron emission tomography. Eur J Clin Pharmacol. 1997;52(2):121-8. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

