Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D2RG4D
|
|||
| Drug Name |
Runcaciguat
|
|||
| Synonyms |
Runcaciguat; 1402936-61-1; Runcaciguat [INN]; 5EZ01YDT5S; UNII-5EZ01YDT5S; (3S)-3-(4-Chloro-3-(((2S,3R)-2-(4-chlorophenyl-4,4,4- trifluoro-3-methylbutanoyl)amino)phenyl)-3- cyclopropylpropanoic acid; (3S)-3-[4-chloro-3-[[(2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methylbutanoyl]amino]phenyl]-3-cyclopropylpropanoic acid; Benzenepropanoic acid, 4-chloro-3-(((2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methyl-1-oxobutyl)amino)-beta-cyclopropyl-, (betaS)-; (3S)-3-(4-chloro-3-{[(2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methylbutanoyl]amino}phenyl)-3-cyclopropylpropanoic acid; CHEMBL4650322; SCHEMBL20075857; GTPL12359; AKOS040742586; BAY1101042; BAY 1101042; BAY-1101042; compound 45 [PMID: 33872507]; AC-37098; MS-29070; HY-109136; CS-0086784; BENZENEPROPANOIC ACID, 4-CHLORO-3-(((2S,3R)-2-(4-CHLOROPHENYL)-4,4,4-TRIFLUORO-3-METHYL-1-OXOBUTYL)AMINO)-.BETA.-CYCLOPROPYL-, (.BETA.S)-; XZ7
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Chronic kidney disease [ICD-11: GB61; ICD-10: N18, N18.9] | Phase 2 | [1] | |
| Non-proliferative diabetic retinopathy [ICD-11: 9B71.00] | Phase 2 | [2] | ||
| Company |
Bayer
|
|||
| Structure |
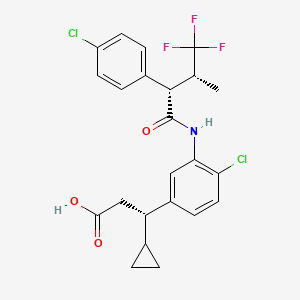 |
Download2D MOL |
||
| Formula |
C23H22Cl2F3NO3
|
|||
| Canonical SMILES |
CC(C(C1=CC=C(C=C1)Cl)C(=O)NC2=C(C=CC(=C2)C(CC(=O)O)C3CC3)Cl)C(F)(F)F
|
|||
| InChI |
InChI=1S/C23H22Cl2F3NO3/c1-12(23(26,27)28)21(14-4-7-16(24)8-5-14)22(32)29-19-10-15(6-9-18(19)25)17(11-20(30)31)13-2-3-13/h4-10,12-13,17,21H,2-3,11H2,1H3,(H,29,32)(H,30,31)/t12-,17+,21+/m1/s1
|
|||
| InChIKey |
NCRMKIWHFXSBGZ-CNBXIYLPSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Soluble guanylate cyclase (GCS) | Target Info | Activator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04507061) A Randomized, Double-blind, Placebo-controlled, Multi-center Study to Assess the Safety and Efficacy of Individually Titrated Oral Doses of Runcaciguat in Subjects With Clinical Diagnosis of Chronic Kidney Disease With Diabetes and/or Hypertension and at Least One Cardiovascular Comorbidity. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT04722991) A Phase 2 Randomized, Placebo-controlled, Double-masked Proof-of-concept Study to Investigate the Efficacy and Safety of Runcaciguat (BAY 1101042) in Patients With Moderately Severe to Severe Non-proliferative Diabetic Retinopathy. U.S.National Institutes of Health. | |||
| REF 3 | Runcaciguat, a novel soluble guanylate cyclase activator, shows renoprotection in hypertensive, diabetic, and metabolic preclinical models of chronic kidney disease. Naunyn Schmiedebergs Arch Pharmacol. 2021 Dec;394(12):2363-2379. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

