Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D67DST
|
|||
| Drug Name |
AD-35
|
|||
| Synonyms |
1531586-58-9; 6'-(2-(1-(Pyridin-2-ylmethyl)piperidin-4-yl)ethyl)spiro[cyClopropane-1,5'-[1,3]dioxolo[4,5-f]isoindol]-7'(6'H)-one; starbld0021420; CHEMBL3949886; SCHEMBL15598869; AD-35; BDBM231544; GLXC-15057; IND-120499; US9346818, I-29; US9346818, I-33; US9346818, I-35
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1] | |
| Company |
Hisun USA Bridgewater, NJ
|
|||
| Structure |
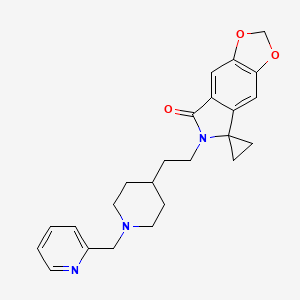 |
Download2D MOL |
||
| Formula |
C24H27N3O3
|
|||
| Canonical SMILES |
C1CN(CCC1CCN2C(=O)C3=CC4=C(C=C3C25CC5)OCO4)CC6=CC=CC=N6
|
|||
| InChI |
InChI=1S/C24H27N3O3/c28-23-19-13-21-22(30-16-29-21)14-20(19)24(7-8-24)27(23)12-6-17-4-10-26(11-5-17)15-18-3-1-2-9-25-18/h1-3,9,13-14,17H,4-8,10-12,15-16H2
|
|||
| InChIKey |
SJXBQKLTADWYTC-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03790982) A Randomized, Double Blind, Placebo Controlled, Parallel-Group 52-week Multicenter Phase II Study to Investigate the Safety, Efficacy and Pharmacokinetics of AD-35 Tablet in Subjects With Mild to Moderate Alzheimer's Disease. U.S.National Institutes of Health. | |||
| REF 2 | Multifunctional Compound AD-35 Improves Cognitive Impairment and Attenuates the Production of TNF-alpha and IL-1beta in an Abeta25-35-induced Rat Model of Alzheimer's Disease. J Alzheimers Dis. 2017;56(4):1403-1417. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

