Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D6N5UC
|
|||
| Drug Name |
ATI-502
|
|||
| Synonyms |
ifidancitinib; UNII-R105E71J13; R105E71J13; A-301; 5-[[2-(4-fluoro-3-methoxy-5-methylanilino)-5-methylpyrimidin-4-yl]amino]-3H-1,3-benzoxazol-2-one; Ifidancitinib [INN]; SCHEMBL342002; CHEMBL4594441; GTPL10638; 2(3H)-Benzoxazolone, 5-((2-((4-fluoro-3-methoxy-5-methylphenyl)amino)-5-methyl-4-pyrimidinyl)amino)-; 5-((2-((4-Fluoro-3-methoxy-5-methylphenyl)amino)-5-methyl-4-pyrimidinyl)amino)-2(3H)-benzoxazolone; 5-((2-(4-Fluoro-3-methoxy-5-methylphenylamino)-5-methylpyrimidin-4-yl)amino)benzo(d)oxazol-2(3H)-one; 5-(2-(4-fluoro-3-methoxy-5-methylphenylamino)-5-methylpyrimidin-4-ylamino)benzo[d]oxazol-2(3h)-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alopecia [ICD-11: ED70; ICD-9: 704.09] | Phase 2 | [1] | |
| Atopic dermatitis [ICD-11: EA80; ICD-10: L20] | Phase 2 | [1] | ||
| Company |
Aclaris Therapeutics
|
|||
| Structure |
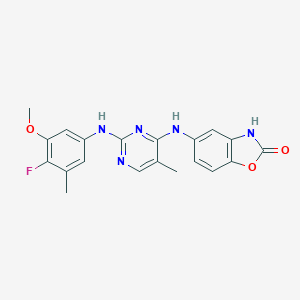 |
Download2D MOL |
||
| Formula |
C20H18FN5O3
|
|||
| Canonical SMILES |
CC1=CC(=CC(=C1F)OC)NC2=NC=C(C(=N2)NC3=CC4=C(C=C3)OC(=O)N4)C
|
|||
| InChI |
1S/C20H18FN5O3/c1-10-6-13(8-16(28-3)17(10)21)24-19-22-9-11(2)18(26-19)23-12-4-5-15-14(7-12)25-20(27)29-15/h4-9H,1-3H3,(H,25,27)(H2,22,23,24,26)
|
|||
| InChIKey |
OYFMQDVLFYKOPZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1236667-40-5
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03759340) ATI-502 Topical Solution for the Treatment of Alopecia Areata (AA), Alopecia Universalis (AU) and Alopecia Totalis (AT). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

