Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DDE6F1
|
|||
| Drug Name |
GSK2292767
|
|||
| Synonyms |
GSK2292767; 1254036-66-2; GSK-2292767; 4M4E8NZ73C; Methanesulfonamide, N-[5-[4-[5-[[(2R,6S)-2,6-dimethyl-4-morpholinyl]methyl]-2-oxazolyl]-1H-indazol-6-yl]-2-methoxy-3-pyridinyl]-, rel-; CHEMBL4434674; PI3Kdelta inhibitor GS2292767; n-[5-[4-(5-{[(2r,6s)-2,6-dimethyl-4-morpholinyl]methyl}-1,3-oxazol-2-yl)-1h-indazol-6-yl]-2-(methyloxy)-3-pyridinyl]methanesulfonamide; N-(5-(4-(5-(((2R,6S)-2,6-Dimethylmorpholino)methyl)oxazol-2-yl)-1H-indazol-6-yl)-2-methoxypyridin-3-yl)methanesulfonamide; Methanesulfonamide, N-(5-(4-(5-(((2R,6S)-2,6-dimethyl-4-morpholinyl)methyl)-2-oxazolyl)-1H-indazol-6-yl)-2-methoxy-3-pyridinyl)-, rel-; N-[5-[4-[5-[[(2S,6R)-2,6-Dimethylmorpholin-4-yl]methyl]-1,3-oxazol-2-yl]-1H-indazol-6-yl]-2-methoxypyridin-3-yl]methanesulfonamide; UNII-4M4E8NZ73C; SCHEMBL173490; C24H28N6O5S; GTPL10633; EX-A994; BCP24323; BDBM50521218; MFCD22572737; s7938; AKOS032945160; CCG-269791; compound 2 [PMID: 30532965]; compound 3 [PMID: 26301626]; AC-32649; HY-15280; CS-0003832; Q27464135; N-{5-[4-(5-{[(2R,6S)-2,6-dimethylmorpholin-4-yl]methyl}-1,3-oxazol-2-yl)-1H-indazol-6-yl]-2-methoxypyridin-3-yl}methanesulfonamide; rel-N-[5-[4-[5-[[(2R,6S)-2,6-Dimethyl-4-morpholinyl]methyl]-2-oxazolyl]-1H-indazol-6-yl]-2-methoxy-3-pyridinyl]methanesulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Phase 1 | [1] | |
| Company |
GlaxoSmithKline
|
|||
| Structure |
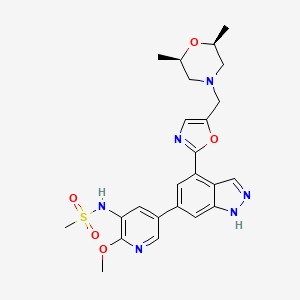 |
Download2D MOL |
||
| Formula |
C24H28N6O5S
|
|||
| Canonical SMILES |
CC1CN(CC(O1)C)CC2=CN=C(O2)C3=C4C=NNC4=CC(=C3)C5=CC(=C(N=C5)OC)NS(=O)(=O)C
|
|||
| InChI |
InChI=1S/C24H28N6O5S/c1-14-11-30(12-15(2)34-14)13-18-9-26-23(35-18)19-5-16(6-21-20(19)10-27-28-21)17-7-22(29-36(4,31)32)24(33-3)25-8-17/h5-10,14-15,29H,11-13H2,1-4H3,(H,27,28)/t14-,15+
|
|||
| InChIKey |
NLUPPCTVKHDVIQ-GASCZTMLSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03045887) A Single-centre, Double-blind (Sponsor Open), Placebo Controlled Two Part Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Single and Repeat Doses of GSK2292767 as a Dry Powder in Healthy Participants Who Smoke Cigarettes. U.S.National Institutes of Health. | |||
| REF 2 | Translation of Inhaled Drug Optimization Strategies into Clinical Pharmacokinetics and Pharmacodynamics Using GSK2292767A, a Novel Inhaled Phosphoinositide 3-Kinase delta Inhibitor. J Pharmacol Exp Ther. 2019 Jun;369(3):443-453. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

