Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DFA0W6
|
|||
| Drug Name |
AKST4290
|
|||
| Synonyms |
Lazucirnon; ALK4290; ALK-4290; AKST4290; 1251528-23-0; Lazucirnon [USAN]; ALK429; AKST-4290; R0T9LLR4TN; BI144807; ALK-429; Lazucirnon (USAN); 2-[[(2R)-1-[1-[(4-chloro-3-methylphenyl)methyl]piperidin-4-yl]-5-oxopyrrolidine-2-carbonyl]amino]-N,N,6-trimethylpyridine-4-carboxamide; 2-((((2R)-1-(1-((4-Chloro-3-methylphenyl)methyl)-4-piperidinyl)-5-oxo-2-pyrrolidinyl)carbonyl)amino)-N,N,6-trimethyl-4-pyridinecarboxamide; 4-Pyridinecarboxamide, 2-((((2R)-1-(1-((4-chloro-3-methylphenyl)methyl)-4-piperidinyl)-5-oxo-2-pyrrolidinyl)carbonyl)amino)-N,N,6-trimethyl-; LAZUCIRNON [INN]; UNII-R0T9LLR4TN; SCHEMBL1668125; CHEMBL3670800; GTPL10653; BDBM123072; GLXC-26875; WHO 11481; AKOS040755379; DB15269; MS-29506; Example 11 [WO2012045803A1]; HY-136788; CS-0133685; D11741; US8742115, 11; 2-{(2R)-1-[1-(4-Chloro-3-methylbenzyl)piperidin-4-yl]-5-oxopyrrolidin-2-carboxamido}-N,N,6-trimethylpyridine-4-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-10: F02.3, G20; ICD-9: 332] | Phase 2 | [1] | |
| Company |
Alkahest
|
|||
| Structure |
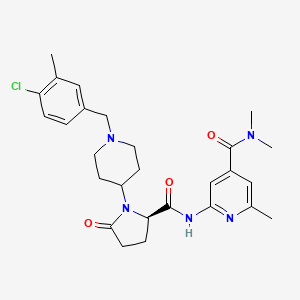 |
Download2D MOL |
||
| Formula |
C27H34ClN5O3
|
|||
| Canonical SMILES |
CC1=CC(=CC(=N1)NC(=O)C2CCC(=O)N2C3CCN(CC3)CC4=CC(=C(C=C4)Cl)C)C(=O)N(C)C
|
|||
| InChI |
InChI=1S/C27H34ClN5O3/c1-17-13-19(5-6-22(17)28)16-32-11-9-21(10-12-32)33-23(7-8-25(33)34)26(35)30-24-15-20(14-18(2)29-24)27(36)31(3)4/h5-6,13-15,21,23H,7-12,16H2,1-4H3,(H,29,30,35)/t23-/m1/s1
|
|||
| InChIKey |
DWKNOLCXIFYNFV-HSZRJFAPSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | C-C chemokine receptor type 3 (CCR3) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| Chemokine signaling pathway | ||||
| Viral carcinogenesis | ||||
| NetPath Pathway | IL3 Signaling Pathway | |||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Reactome | Chemokine receptors bind chemokines | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| IL1 and megakaryotyces in obesity | ||||
| IL-3 Signaling Pathway | ||||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04369430) A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of AKST4290 in Subjects With Parkinson's Disease on Stable Dopaminergic Treatment. U.S.National Institutes of Health. | |||
| REF 2 | SAFETY AND THERAPEUTIC EFFECTS OF ORALLY ADMINISTERED AKST4290 IN NEWLY DIAGNOSED NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. Retina. 2022 Jun 1;42(6):1038-1046. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

