Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DIK8W5
|
|||
| Drug Name |
MGB-BP-3
|
|||
| Synonyms |
1000277-08-6; MGB-BP3; UNII-532PWU9738; 532PWU9738; J2.725.402K; (E)-1-methyl-4-(1-methyl-4-(4-(2-(quinolin-3-yl)vinyl)benzamido)-1H-pyrrole-2-carboxamido)-N-(2-morpholinoethyl)-1H-pyrrole-2-carboxamide; 1-methyl-N-[1-methyl-5-(2-morpholin-4-ylethylcarbamoyl)pyrrol-3-yl]-4-[[4-[(E)-2-quinolin-3-ylethenyl]benzoyl]amino]pyrrole-2-carboxamide; 4-((4-(4-((E)-2-(3-Quinolyl)vinyl)benzoylamino)-1-methyl-1H-pyrrole-2-yl)carbonylamino)-1-methyl-N-(2-morpholinoethyl)-1H-pyrrole-2-carboxamide; 4-[[4-[4-[(E)-2-(3-Quinolyl)vinyl]benzoylamino]-1-methyl-1H-pyrrole-2-yl]carbonylamino]-1-methyl-N-(2-morpholinoethyl)-1H-pyrrole-2-carboxamide; CHEMBL236345; SCHEMBL2398755; GTPL10996; HY-U00035; ZINC28864451; CS-6785; DB12892; SB19161; compound 50 [PMID: 17960927]; Q27261055; 1H-Pyrrole-2-carboxamide, 1-methyl-N-(1-methyl-5-(((2-(4-morpholinyl)ethyl)amino)carbonyl)-1H-pyrrol-3-yl)-4-((4-((1E)-2-(3-quinolinyl)ethenyl)benzoyl)amino)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Clostridium infection [ICD-11: 1A04; ICD-10: A04.7] | Phase 2 | [1] | |
| Company |
MGB Biopharma
|
|||
| Structure |
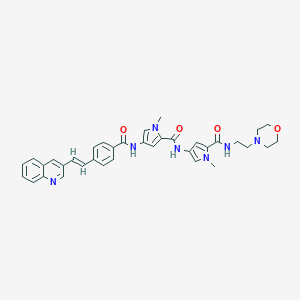 |
Download2D MOL |
||
| Formula |
C36H37N7O4
|
|||
| Canonical SMILES |
CN1C=C(C=C1C(=O)NC2=CN(C(=C2)C(=O)NCCN3CCOCC3)C)NC(=O)C4=CC=C(C=C4)C=CC5=CC6=CC=CC=C6N=C5
|
|||
| InChI |
1S/C36H37N7O4/c1-41-24-30(20-32(41)35(45)37-13-14-43-15-17-47-18-16-43)40-36(46)33-21-29(23-42(33)2)39-34(44)27-11-9-25(10-12-27)7-8-26-19-28-5-3-4-6-31(28)38-22-26/h3-12,19-24H,13-18H2,1-2H3,(H,37,45)(H,39,44)(H,40,46)/b8-7+
|
|||
| InChIKey |
OEKXCVYZBVOWBR-BQYQJAHWSA-N
|
|||
| CAS Number |
CAS 1000277-08-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Deoxyribonucleic acid (Bact DNA) | Target Info | Binder | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03824795) Study to Assess Safety, Tolerability and Efficacy of Incremental Doses of MGB-BP-3 in Patients With CDAD. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of MGB Biopharma. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

