Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DJ48HT
|
|||
| Drug Name |
Iptacopan
|
|||
| Synonyms |
LNP-023; UNII-8E05T07Z6W; 8E05T07Z6W; 1644670-37-0; 4-((2S,4S)-4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl)benzoic acid; 4-[(2~{S},4~{S})-4-ethoxy-1-[(5-methoxy-7-methyl-1~{H}-indol-4-yl)methyl]piperidin-2-yl]benzoic acid; 4-[(2S,4S)-4-ethoxy-1-[(5-methoxy-7-methyl-1H-indol-4-yl)methyl]piperidin-2-yl]benzoic acid; Iptacopan [INN]; CHEMBL4594448; SCHEMBL16400416; GTPL10710; US9682968, Example-26a; BDBM160475; ZINC223246892; compound 41 [PMID: 32073845]; HY-127105; CS-0093107; 4-((2S,4S)-(4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl))benzoic acid; Benzoic acid, 4-((2S,4S)-4-ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)-2-piperidinyl)-; JGQ
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Paroxysmal nocturnal haemoglobinuria [ICD-11: 3A21.0; ICD-10: D59.5] | Approved | [1] | |
| IgA nephropathy [ICD-11: MF8Y] | Phase 3 | [2] | ||
| Company |
Novartis
|
|||
| Structure |
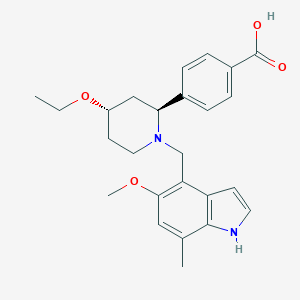 |
Download2D MOL |
||
| Formula |
C25H30N2O4
|
|||
| Canonical SMILES |
CCOC1CCN(C(C1)C2=CC=C(C=C2)C(=O)O)CC3=C(C=C(C4=C3C=CN4)C)OC
|
|||
| InChI |
1S/C25H30N2O4/c1-4-31-19-10-12-27(22(14-19)17-5-7-18(8-6-17)25(28)29)15-21-20-9-11-26-24(20)16(2)13-23(21)30-3/h5-9,11,13,19,22,26H,4,10,12,14-15H2,1-3H3,(H,28,29)/t19-,22-/m0/s1
|
|||
| InChIKey |
RENRQMCACQEWFC-UGKGYDQZSA-N
|
|||
| CAS Number |
CAS 1644670-37-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Complement factor B (CFB) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Complement and coagulation cascades | |||
| Staphylococcus aureus infection | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Reactome | Alternative complement activation | |||
| Activation of C3 and C5 | ||||
| Regulation of Complement cascade | ||||
| WikiPathways | Complement and Coagulation Cascades | |||
| Human Complement System | ||||
| Complement cascade | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 218276 | |||
| REF 2 | ClinicalTrials.gov (NCT04578834) Study of Efficacy and Safety of LNP023 in Primary IgA Nephropathy Patients (APPLAUSE-IgAN). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

