Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DNM79H
|
|||
| Drug Name |
DS-3201b
|
|||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | T-cell leukaemia [ICD-11: 2A90; ICD-10: C86] | Phase 2 | [1] | |
| Small-cell lung cancer [ICD-11: 2C25.Y] | Phase 1/2 | [2] | ||
| Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C91.0] | Phase 1 | [3] | ||
| Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Phase 1 | [3] | ||
| Renal cell carcinoma [ICD-11: 2C90; ICD-10: C64; ICD-9: 189] | Phase 1 | [4] | ||
| Urothelial carcinoma [ICD-11: 2C92.0] | Phase 1 | [4] | ||
| Company |
Daiichi Sankyo
|
|||
| Structure |
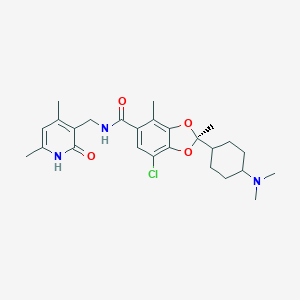 |
Download2D MOL |
||
| Formula |
C26H34ClN3O4
|
|||
| Canonical SMILES |
CC1=CC(=C(C(=O)N1)CNC(=O)C2=CC(=C3C(=C2C)OC(O3)(C)C4CCC(CC4)N(C)C)Cl)C
|
|||
| InChI |
1S/C26H34ClN3O4/c1-14-11-15(2)29-25(32)20(14)13-28-24(31)19-12-21(27)23-22(16(19)3)33-26(4,34-23)17-7-9-18(10-8-17)30(5)6/h11-12,17-18H,7-10,13H2,1-6H3,(H,28,31)(H,29,32)/t17?,18?,26-/m1/s1
|
|||
| InChIKey |
SSDRNUPMYCFXGM-ZZHSESOFSA-N
|
|||
| CAS Number |
CAS 1809336-39-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Enhancer of zeste homolog 1 (EZH1) | Target Info | Inhibitor | [5] |
| Enhancer of zeste homolog 2 (EZH2) | Target Info | Inhibitor | [5] | |
| KEGG Pathway | MicroRNAs in cancer | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Reactome | PRC2 methylates histones and DNA | |||
| Oxidative Stress Induced Senescence | ||||
| PKMTs methylate histone lysines | ||||
| WikiPathways | Interactome of polycomb repressive complex 2 (PRC2) | |||
| Endoderm Differentiation | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| Histone Modifications | ||||
| Cell Differentiation - meta | ||||
| miRs in Muscle Cell Differentiation | ||||
| miR-targeted genes in muscle cell - TarBase | ||||
| miR-targeted genes in lymphocytes - TarBase | ||||
| miR-targeted genes in epithelium - TarBase | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04102150) Valemetostat Tosylate (DS-3201b) Phase 2 Study in Relapsed or Refractory Adult T-cell Leukemia/Lymphoma. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT03879798) DS-3201b and Irinotecan for Patients With Recurrent Small Cell Lung Cancer. U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT03110354) DS-3201b for Acute Myelogenous Leukemia (AML) or Acute Lymphocytic Leukemia (ALL). U.S. National Institutes of Health. | |||
| REF 4 | ClinicalTrials.gov (NCT04388852) DS3201 and Ipilimumab for the Treatment of Metastatic Prostate, Urothelial and Renal Cell Cancers. U.S. National Institutes of Health. | |||
| REF 5 | Development of new agents for peripheral T-cell lymphoma. Expert Opin Biol Ther. 2019 Mar;19(3):197-209. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

