Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DP7GK8
|
|||
| Drug Name |
ABBV-951
|
|||
| Synonyms |
Foslevodopa; UNII-37NQZ0J76I; 37NQZ0J76I; 3-hydroxy-O-phosphono-L-tyrosine; 101141-95-1; 97321-87-4; Dopa 4-phosphate; Levodopa-4'-monophosphate; 3-Hydroxy-O-phosphonotyrosine; Foslevodopa (JAN/USAN/INN); CHEMBL4594379; SCHEMBL17685899; DTXSID70905957; HY-109132; CS-0086774; D11839; (S)-2-amino-3-(3-hydroxy-4-(phosphonooxy)phenyl)propanoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-10: F02.3, G20; ICD-9: 332] | Phase 3 | [1] | |
| Company |
AbbVie
|
|||
| Structure |
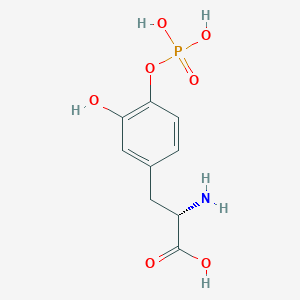 |
Download2D MOL |
||
| Formula |
C9H12NO7P
|
|||
| Canonical SMILES |
C1=CC(=C(C=C1CC(C(=O)O)N)O)OP(=O)(O)O
|
|||
| InChI |
1S/C9H12NO7P/c10-6(9(12)13)3-5-1-2-8(7(11)4-5)17-18(14,15)16/h1-2,4,6,11H,3,10H2,(H,12,13)(H2,14,15,16)/t6-/m0/s1
|
|||
| InChIKey |
YNDMEEULGSTYJT-LURJTMIESA-N
|
|||
| CAS Number |
CAS 97321-87-4
|
|||
| PubChem Compound ID | ||||
| ADReCS Drug ID | BADD_D01274 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dopamine receptor (DR) | Target Info | Activator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04750226) Study To Assess Adverse Events and Change in Disease Activity Of 24-hour Continuous Subcutaneous Infusion Of ABBV-951 In Adult Participants With Advanced Parkinson's Disease. U.S. National Institutes of Health. | |||
| REF 2 | Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson's Disease. Ann Neurol. 2021 Jul;90(1):52-61. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

