Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DQ15ZM
|
|||
| Drug Name |
Zunsemetinib
|
|||
| Synonyms |
zunsemetinib; ATI-450; (R)-Zunsemetinib; Zunsemetinib [INN]; ATI450; CDD450; Zunsemetinib [USAN]; CDD-450; AX2VWG0ZCR; 1640282-42-3; 1639791-42-6; 3-chloro-4-[(3,5-difluoropyridin-2-yl)methoxy]-1-[2-[2-(2-hydroxypropan-2-yl)pyrimidin-4-yl]-5-methylpyridin-4-yl]-6-methylpyridin-2-one; 3-chloro-4-[(3,5-difluoropyridin-2-yl)methoxy]-1-{2-[2-(2-hydroxypropan-2-yl)pyrimidin-4-yl]-5-methylpyridin-4-yl}-6-methylpyridin-2-one; UNII-AX2VWG0ZCR; Zunsemetinib M-atropisomer; Zunsemetinib [USAN:INN]; Ati 450; ATI 450 [WHO-DD]; CHEMBL3704901; SCHEMBL16279876; GTPL11681; BDBM175242; EX-A6292; WHO 11983; AKOS040756965; HY-139553A; MS-29543; HY-139553; CS-0204147; CS-0374185; US9115089, 49; (-)-3-Chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl[1(2H),4'-bipyridin]-2-one; (1(2H),4'-Bipyridin)-2-one, 3-chloro-4-((3,5-difluoro-2-pyridinyl)methoxy)-2'-(2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl)-5',6-dimethyl-, (-)-; (2'S)-3-Chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl[1(2H),4'-bipyridin]-2-one; (P)-(3-chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-[1,4'-bipyridin]-2-one); (p)-3-Chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-(1,4'-bipyridin)-2-one; [1(2H),4'-Bipyridin]-2-one, 3-chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl-, (2'S)-; 1640282-44-5; 3-Chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-[1,4'-bipyridin]-2-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Cryopyrin-associated periodic syndrome [ICD-11: 4A60.1] | Phase 2 | [1] | |
| Hidradenitis suppurativa [ICD-11: ED92.0; ICD-10: L73.2] | Phase 2 | [2] | ||
| Psoriatic arthritis [ICD-11: FA21; ICD-9: 696] | Phase 2 | [3] | ||
| Rheumatoid arthritis [ICD-11: FA20; ICD-9: 729] | Phase 2 | [4] | ||
| Company |
Aclaris Therapeutics
|
|||
| Structure |
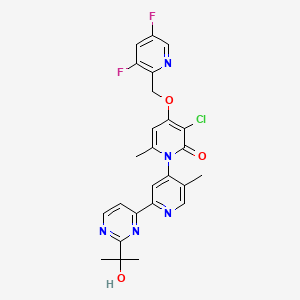 |
Download2D MOL |
||
| Formula |
C25H22ClF2N5O3
|
|||
| Canonical SMILES |
CC1=CC(=C(C(=O)N1C2=CC(=NC=C2C)C3=NC(=NC=C3)C(C)(C)O)Cl)OCC4=C(C=C(C=N4)F)F
|
|||
| InChI |
InChI=1S/C25H22ClF2N5O3/c1-13-10-30-18(17-5-6-29-24(32-17)25(3,4)35)9-20(13)33-14(2)7-21(22(26)23(33)34)36-12-19-16(28)8-15(27)11-31-19/h5-11,35H,12H2,1-4H3
|
|||
| InChIKey |
FQPQMJULRZINPV-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04524858) A Phase 2a, Open-Label, Single-Arm Study to Investigate the Safety and Efficacy of ATI-450 for the Maintenance of Remission in Patients With Cryopyrin-Associated Periodic Syndrome (CAPS) Previously Managed With Anti-IL-1 Therapy. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT05216224) A Phase 2a, Randomized, Double-blind, Placebo-controlled Study to Investigate the Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of ATI-450 vs Placebo in Patients With Moderate to Severe HS. U.S.National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT05511519) A Phase 2a, Randomized, Double-blind, Placebo-controlled Study to Investigate the Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Zunsemetinib vs Placebo in Patients With Moderate-to-Severe Active Psoriatic Arthritis. U.S.National Institutes of Health. | |||
| REF 4 | ClinicalTrials.gov (NCT04247815) A Phase 2a, Randomized, Investigator and Patient-blind, Sponsor-unblinded, Parallel Group, Placebo-controlled Study of ATI-450 Plus Methotrexate (MTX) vs MTX Alone in Patients With Moderate to Severe Active Rheumatoid Arthritis. U.S.National Institutes of Health. | |||
| REF 5 | Clinical pipeline report, company report or official report of Aclaris Therapeutics | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

