Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DUH2E6
|
|||
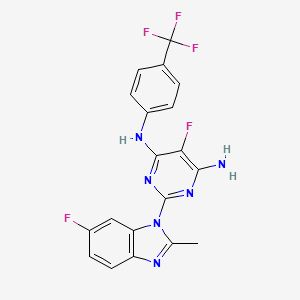
| Drug Name |
Unesbulin
|
|||
| Synonyms |
PTC596; 1610964-64-1; Unesbulin; 5-Fluoro-2-(6-fluoro-2-methyl-1H-benzo[d]imidazol-1-yl)-N4-(4-(trifluoromethyl)phenyl)pyrimidine-4,6-diamine; PTC-596; BMI1 inhibitor PTC596; Unesbulin [USAN]; Z4HZ70S62Q; PTC 596; 5-fluoro-2-(6-fluoro-2-methylbenzimidazol-1-yl)-4-N-[4-(trifluoromethyl)phenyl]pyrimidine-4,6-diamine; 5-fluoranyl-2-(6-fluoranyl-2-methyl-benzimidazol-1-yl)-~{N}4-[4-(trifluoromethyl)phenyl]pyrimidine-4,6-diamine; 5-fluoro-2-(6-fluoro-2-methyl-1H-benzimidazol-1-yl)-N4-[4-(trifluoromethyl)phenyl]-4,6-pyrimidinediamine; 5-Fluoro-2-(6-fluoro-2-methyl-1H-benzo(d)imidazole-1-yl)-N4-(4-(trifluoromethyl)phenyl)pyrimidine-4,6-diamine; 5-FLUORO-2-(6-FLUORO-2-METHYL-1H-BENZIMIDAZOL-1-YL)-N4-(4-(TRIFLUOROMETHYL)PHENYL)-4,6-PYRIMIDINEDIAMINE; UNESBULIN [INN]; UNESBULIN [WHO-DD]; UNII-Z4HZ70S62Q; CHEMBL4594353; SCHEMBL15741319; TWLWOOPCEXYVBE-UHFFFAOYSA-N; C19H13F5N6; BCP33231; EX-A3263; PTC-596; PTC 596; s8820; WHO 11570; AKOS030528004; AC-32587; BS-15871; HY-112041; CS-0042474; D83695; 4,6-PYRIMIDINEDIAMINE, 5-FLUORO-2-(6-FLUORO-2-METHYL-1H-BENZIMIDAZOL-1-YL)-N4-(4-(TRIFLUOROMETHYL)PHENYL)-; SOZ
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Leiomyosarcoma [ICD-11: 2B58] | Phase 2/3 | [1] | |
| Company |
PTC Therapeutics South Plainfield, NJ
|
|||
| Structure |
 |
Download2D MOL |
||
| Formula |
C19H13F5N6
|
|||
| Canonical SMILES |
CC1=NC2=C(N1C3=NC(=C(C(=N3)NC4=CC=C(C=C4)C(F)(F)F)F)N)C=C(C=C2)F
|
|||
| InChI |
InChI=1S/C19H13F5N6/c1-9-26-13-7-4-11(20)8-14(13)30(9)18-28-16(25)15(21)17(29-18)27-12-5-2-10(3-6-12)19(22,23)24/h2-8H,1H3,(H3,25,27,28,29)
|
|||
| InChIKey |
TWLWOOPCEXYVBE-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Polycomb complex protein BMI-1 (BMI1) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05269355) A Phase 2/3 Study to Evaluate the Efficacy and Safety of Unesbulin in Unresectable or Metastatic, Relapsed or Refractory Leiomyosarcoma. U.S.National Institutes of Health. | |||
| REF 2 | Rationale for Combining the BCL2 Inhibitor Venetoclax with the PI3K Inhibitor Bimiralisib in the Treatment of IDH2- and FLT3-Mutated Acute Myeloid Leukemia. Int J Mol Sci. 2022 Oct 20;23(20):12587. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

