Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DW7OF1
|
|||
| Drug Name |
ORP-101
|
|||
| Synonyms |
Buprenorphine dimer; ORP-101; SE6KE496VO; UNII-SE6KE496VO; 1820753-68-1; 6,14-Ethenomorphinan-7-methanol, 3,3'-(1,2-ethanediylbis(oxy))bis(17-(cyclopropylmethyl)-alpha-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-6-methoxy-alpha-methyl-, (alphaS,5alpha,7alpha)-(alpha'S,5'alpha,7'alpha)-; CHEMBL4594402; SCHEMBL19231198; EX-A6870; 6,14-ETHENOMORPHINAN-7-METHANOL, 3,3'-(1,2-ETHANEDIYLBIS(OXY))BIS(17-(CYCLOPROPYLMETHYL)-.ALPHA.-(1,1-DIMETHYLETHYL)-4,5-EPOXY-18,19-DIHYDRO-6-METHOXY-.ALPHA.-METHYL-, (.ALPHA.S,5.ALPHA.,7.ALPHA.)-(.ALPHA.'S,5'.ALPHA.,7'.ALPHA.)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Irritable bowel syndrome [ICD-11: DD91.0; ICD-10: K55-K64, K58; ICD-9: 564.1, 787.91] | Phase 2 | [1] | |
| Company |
OrphoMed
|
|||
| Structure |
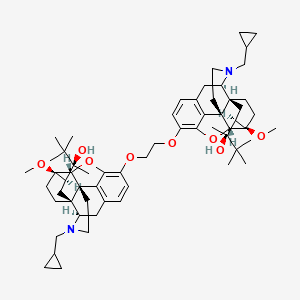 |
Download2D MOL
|
||
| Formula |
C60H84N2O8
|
|||
| Canonical SMILES |
CC(C)(C)C(C)(C1CC23CCC1(C4C25CCN(C3CC6=C5C(=C(C=C6)OCCOC7=C8C9=C(CC1C23C9(CCN1CC1CC1)C(O8)C(CC2)(C(C3)C(C)(C(C)(C)C)O)OC)C=C7)O4)CC1CC1)OC)O
|
|||
| InChI |
InChI=1S/C60H84N2O8/c1-51(2,3)53(7,63)41-31-55-19-21-59(41,65-9)49-57(55)23-25-61(33-35-11-12-35)43(55)29-37-15-17-39(47(69-49)45(37)57)67-27-28-68-40-18-16-38-30-44-56-20-22-60(66-10,42(32-56)54(8,64)52(4,5)6)50-58(56,46(38)48(40)70-50)24-26-62(44)34-36-13-14-36/h15-18,35-36,41-44,49-50,63-64H,11-14,19-34H2,1-10H3/t41-,42-,43-,44-,49-,50-,53+,54+,55-,56-,57+,58+,59-,60-/m1/s1
|
|||
| InChIKey |
LEQOVFCHMOTJKU-WJVXOHEGSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Opioid receptor kappa (OPRK1) | Target Info | Inhibitor | [2] |
| Opioid receptor mu (MOP) | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Estrogen signaling pathway | ||||
| Morphine addiction | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Enkephalin release | ||||
| Opioid prodynorphin pathway | ||||
| Pathway Interaction Database | IL4-mediated signaling events | |||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | TCR Signaling Pathway | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Peptide GPCRs | ||||
| Opioid Signalling | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04129619) A Double-Blind, Placebo-Controlled, Phase 2, Responsive Adaptive Randomization Study of ORP-101 in Patients With Irritable Bowel Syndrome With Diarrhea (IBS-D). U.S.National Institutes of Health. | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2023. Adis Insight | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

