Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T20761 | ||||
| Target Name | Vascular endothelial growth factor A (VEGFA) | ||||
| Synonyms | Vascular permeability factor; VPF; VEGF-A; VEGF | ||||
| Target Type | Successful | ||||
| Gene Name | VEGFA | ||||
| Biochemical Class | Growth factor | ||||
| UniProt ID | VEGFA_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Rectal cancer | ||||
| Example drug | Aflibercept | Approved | [1], [2], [3] | ||
| Tissue | Rectal colon tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 1.2 Z-score: 2.26 P-value: 2.07E-03 |
||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: 0.97 Z-score: 4.06 P-value: 1.28E-05 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
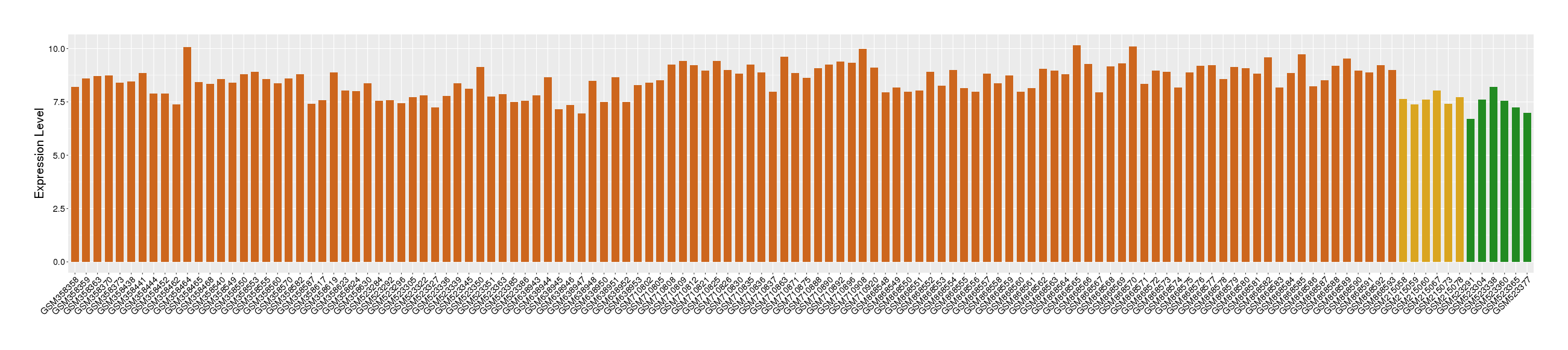
|
|||||
| Disease | Lateral sclerosis | ||||
| Example drug | SNN-0029 | Phase 1/2 | [2], [3], [4] | ||
| Tissue | Cervical spinal cord | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 0.15 Z-score: 0.47 P-value: 3.61E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
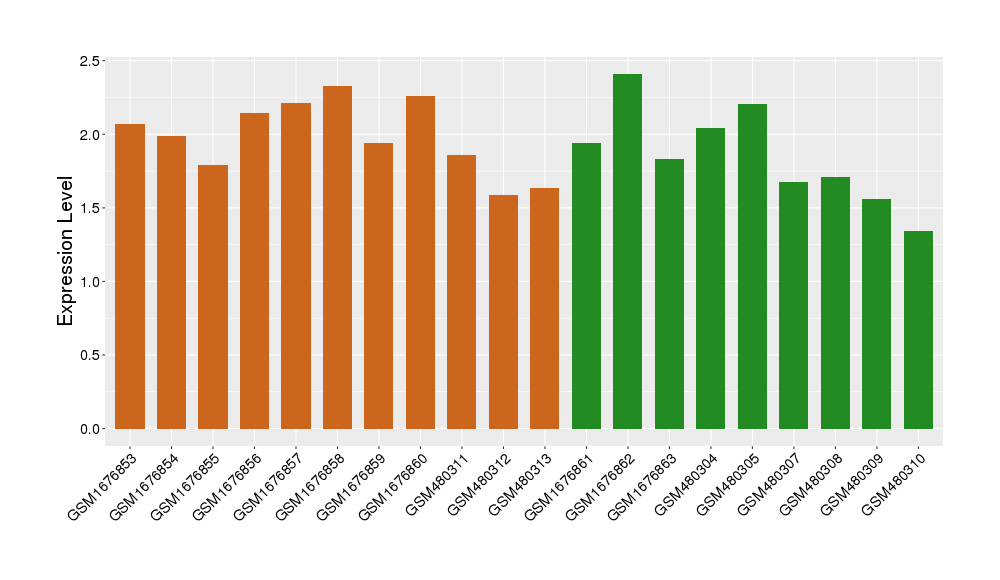
|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
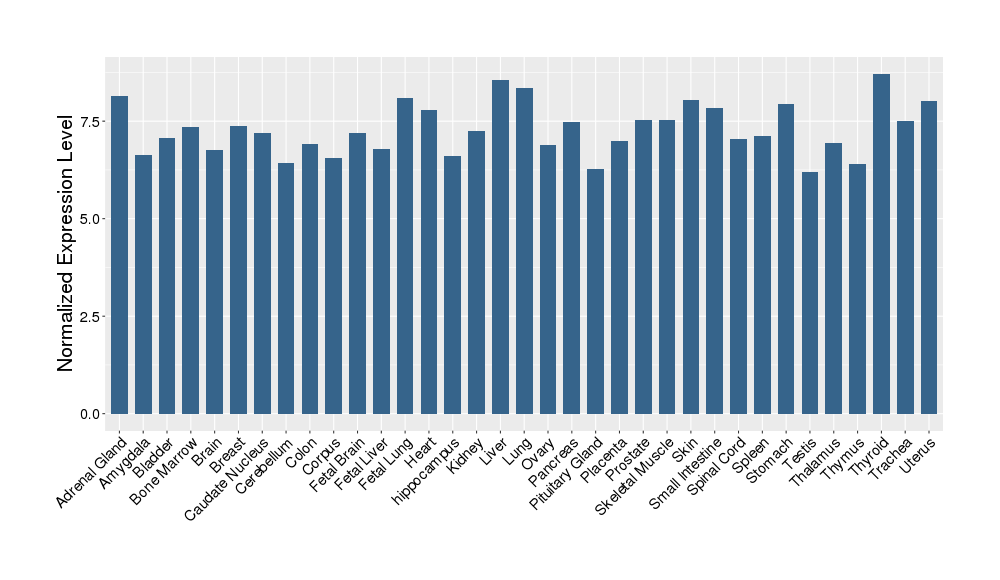
|
|||||
| References | |||||
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 2 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
| REF 4 | ClinicalTrials.gov (NCT01384162) An Open Label, Safety and Tolerability Continuation Study of Intracerebroventricular Administration of sNN0029 to Patients With Amyotrophic Lateral Sclerosis. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

