Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T63967 | ||||
| Target Name | Neuronal acetylcholine receptor alpha-4/beta-2 (CHRNA4/B2) | ||||
| Synonyms | CHRN; nAChR | ||||
| Target Type | Successful | ||||
| Gene Name | CHRNA4-CHRNB2 | ||||
| Biochemical Class | Neurotransmitter receptor | ||||
| UniProt ID | ACHA4_HUMAN||ACHB2_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Schizophrenia | ||||
| Example drug | Ispronicline | Discontinued in Phase 2 | [1], [2], [3] | ||
| Tissue | Pre-frontal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 0.02 Z-score: 0.15 P-value: 7.65E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
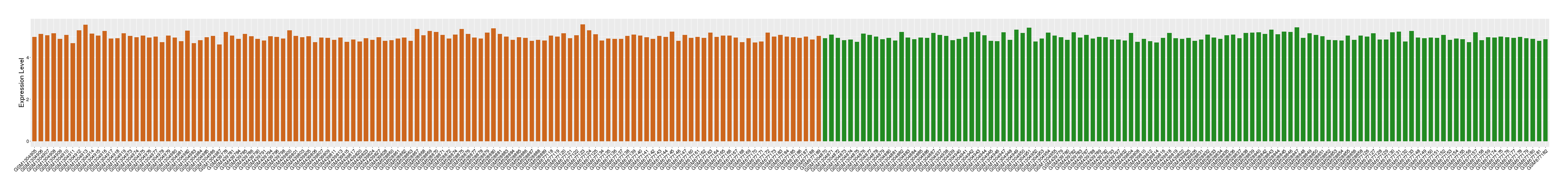
|
|||||
| Disease | Schizophrenia | ||||
| Example drug | Ispronicline | Discontinued in Phase 2 | [1], [2], [3] | ||
| Tissue | Superior temporal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.01 Z-score: -0.11 P-value: 9.89E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
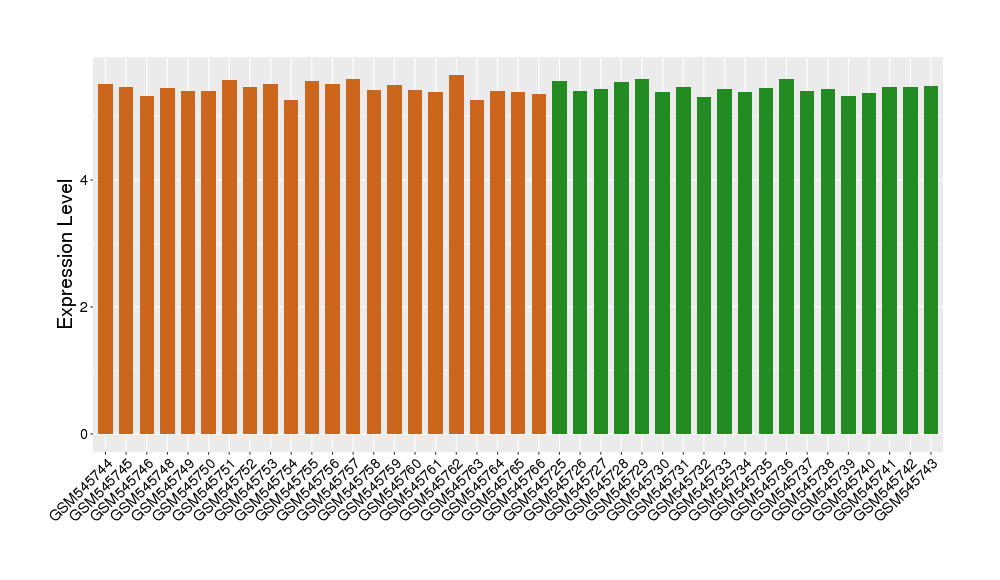
|
|||||
| Disease | Alzheimer's disease | ||||
| Example drug | AZD-1446 | Phase 2 | [2], [3], [4] | ||
| Tissue | Entorhinal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.15 Z-score: -0.61 P-value: 3.51E-10 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Irritable bowel syndrome | ||||
| Example drug | TC-6499-12 | Phase 2 | [2], [3], [5] | ||
| Tissue | Rectal colon tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -3.94E-03 Z-score: -0.02 P-value: 1.09E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
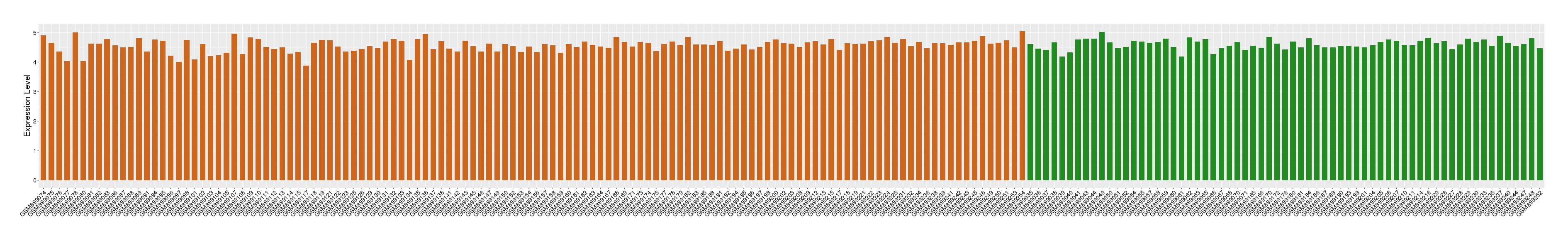
|
|||||
| Disease | Parkinson's disease | ||||
| Example drug | SIB-1508Y | Terminated | [2], [3], [6], [7] | ||
| Tissue | Substantia nigra tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.12 Z-score: -0.71 P-value: 1.04E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
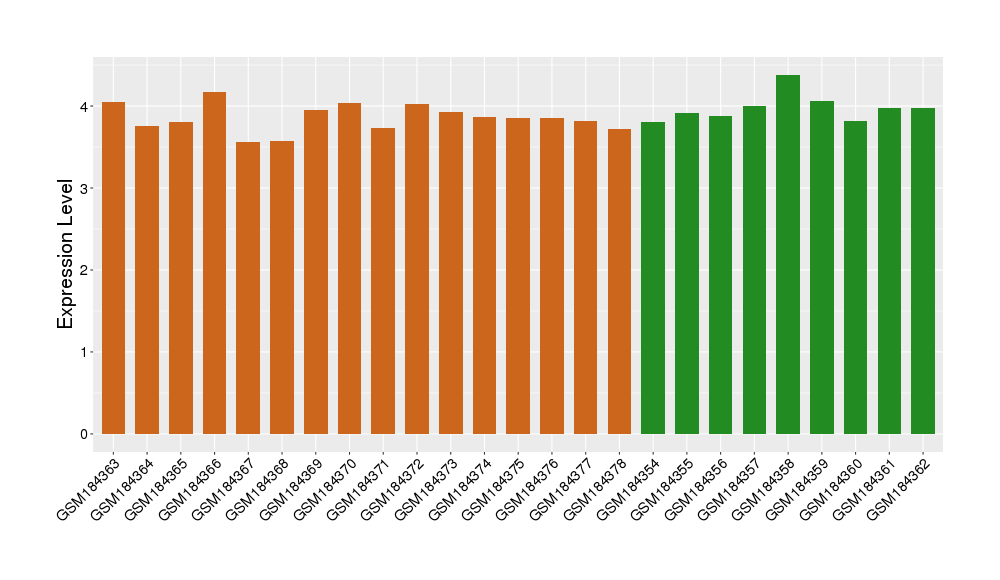
|
|||||
| Disease | Eosinophilic gastritis | ||||
| Tissue | Gastric antrum tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: 0.19 Z-score: 1.11 P-value: 3.06E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
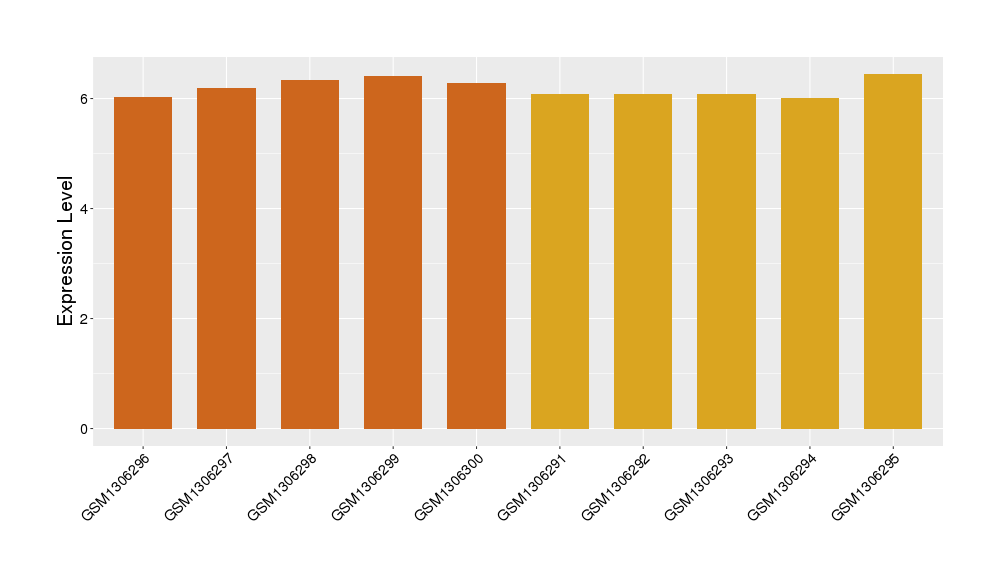
|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
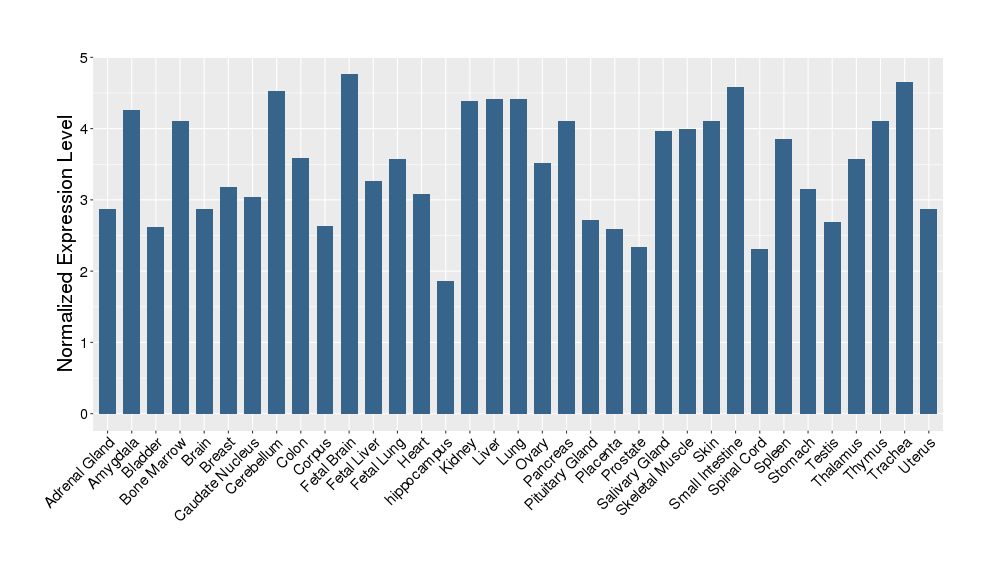
|
|||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011083) | ||||
| REF 2 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
| REF 4 | ClinicalTrials.gov (NCT01012375) Study to Investigate the Efficacy and Tolerability of AZD1446 in Adult ADHD Patients.. U.S. National Institutes of Health. | ||||
| REF 5 | ClinicalTrials.gov (NCT01149200) Proof-of-Principle Study of TC-6499 in the Treatment of Constipation Predominant Irritable Bowel Syndrome (IBS). U.S. National Institutes of Health. | ||||
| REF 6 | Pharmacological characterization of (S)-(2)-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine HCl (SIB-1508Y, Altinicline), a novel nicotinic acetylcholine receptor agonist. Brain Res. 2008 Oct 9;1234:16-24. | ||||
| REF 7 | Recombinant human receptors and functional assays in the discovery of altinicline (SIB-1508Y), a novel acetylcholine-gated ion channel (nAChR) agonist. Pharm Acta Helv. 2000 Mar;74(2-3):125-30. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

