Prodrug Information
| Prodrug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Prodrug ID |
D6OZP9
|
|||||
| Prodrug Name |
Fostemsavir
|
|||||
| Synonyms |
BMS-663068; BMS-663068 free acid; BMS 663068; Fostemsavir(BMS-663068); UNII-97IQ273H4L; Fostemsavir [USAN]; 97IQ273H4L; 864953-29-7(free base); Fostemsavir (USAN); Rukobia; fostemsavir & N6; fostemsavir & PG16; fostemsavir & 4Dm2m; fostemsavir & VRC03; fostemsavir & CH106; fostemsavir & 35O22; fostemsavir & PGT128; fostemsavir & 3BNC117; fostemsavir & PG16-iMab; fostemsavir & PGT128-iMab; fostemsavir & VRC07-523; SCHEMBL754395; Fostemsavir; BMS-663068; CHEMBL3301594
Click to Show/Hide
|
|||||
| Indication | Human immunodeficiency virus-1 infection [ICD-11: 1C62; ICD-10: B20-B24; ICD-9: 42] | Approved | [1] | |||
| Activation |
Prodrug 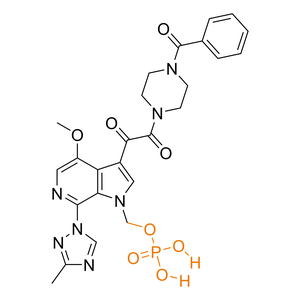
|

|
Parent Drug 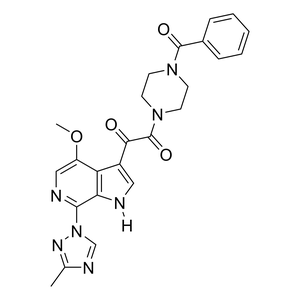
|
|||
| 2D MOL 3D MOL | 2D MOL 3D MOL | |||||
|
(1) Bioconversion Enzyme:
Alkaline phosphatase
(EC 3.1)
|
[2] | |||||
| Prodrug Strategy |
Classical prodrug strategy
[Carrier linked prodrug]
|
|||||
| Improved property |
Increase solubility; Improve stability
|
[4] | ||||
| Description |
Fostemsavir improved aqueous solubility and stability of Temsavir under acidic conditions.
|
[3] | ||||
| Formula |
C25H26N7O8P
|
|||||
| Canonical SMILES |
CC1=NN(C=N1)C2=NC=C(C3=C2N(C=C3C(=O)C(=O)N4CCN(CC4)C(=O)C5=CC=CC=C5)COP(=O)(O)O)OC
|
|||||
| InChI |
1S/C25H26N7O8P/c1-16-27-14-32(28-16)23-21-20(19(39-2)12-26-23)18(13-31(21)15-40-41(36,37)38)22(33)25(35)30-10-8-29(9-11-30)24(34)17-6-4-3-5-7-17/h3-7,12-14H,8-11,15H2,1-2H3,(H2,36,37,38)
|
|||||
| InChIKey |
SWMDAPWAQQTBOG-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 864953-29-7
|
|||||
| PubChem Compound ID | ||||||
| Parent Drug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Parent Drug ID |
DJPE52
|
|||||
| Parent Drug Name |
Temsavir
|
|||||
| Synonyms |
BMS-626529; 701213-36-7; BMS 626529; UNII-4B6J53W8N3; Temsavir (BMS-626529); 4B6J53W8N3; Temsavir [USAN:INN]; BMS626529; Temsavir (USAN); TemsavirBMS-626529; SCHEMBL760768; CHEMBL3301620; DTXSID20462146; C24H23N7O4; BCP09613; EX-A2034; 3501AH; BDBM50236759; MFCD22665723; s6625; ZINC34815611; AKOS025396462; CS-0938; DB14675; SB19162; NCGC00379018-02; AC-30242; AS-35292; DA-35014; HY-15440; FT-0720945; A14148; C21558; D10734; W-5969; Q27259369; 83J;
Click to Show/Hide
|
|||||
| Formula |
C24H23N7O4
|
|||||
| Canonical SMILES |
CC1=NN(C=N1)C2=NC=C(C3=C2NC=C3C(=O)C(=O)N4CCN(CC4)C(=O)C5=CC=CC=C5)OC
|
|||||
| InChI |
1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3
|
|||||
| InChIKey |
QRPZBKAMSFHVRW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 701213-36-7
|
|||||
| PubChem Compound ID | ||||||
| Target and Pathway | Top | |||||
|---|---|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Envelope glycoprotein gp120 (HIV gp120) | Target Info | Binder | [4] | ||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2020 | |||||
| REF 2 | In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012 Jul;56(7):3498-507. | |||||
| REF 3 | Inhibitors of HIV-1 Attachment: The Discovery and Development of Temsavir and its Prodrug Fostemsavir. J Med Chem. 2018 Jan 11;61(1):62-80. | |||||
| REF 4 | Discovery of the Human Immunodeficiency Virus Type 1 (HIV-1) Attachment Inhibitor Temsavir and Its Phosphonooxymethyl Prodrug Fostemsavir. J Med Chem. 2018 Jul 26;61(14):6308-6327. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

