Prodrug Information
| Prodrug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Prodrug ID |
DF0PV9
|
|||||
| Prodrug Name |
Latanoprost
|
|||||
| Synonyms |
AR-202; AT-3016; CHEMBL1051; Catioprost; L-PPDS; Latanoprost, (+/-)-; Latanoprost, ethanol solution; Nova-21027; PHXA-41; PhXA 41; PhXA34; PhXA41; T-2345; Xalatan; latanoprost (isopropyl ester); latanoprostum
Click to Show/Hide
|
|||||
| Indication | Glaucoma/ocular hypertension [ICD-11: 9C61; ICD-9: 365] | Approved | [1] | |||
| Activation |
Prodrug 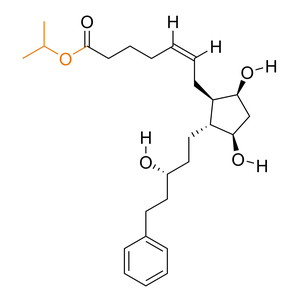
|

|
Parent Drug 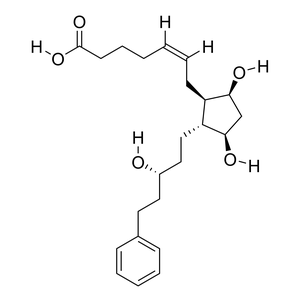
|
|||
| 2D MOL 3D MOL | 2D MOL 3D MOL | |||||
|
(1) Bioconversion Enzyme:
Esterase in corneal
(EC 3.1)
|
[2] | |||||
| Prodrug Strategy |
Classical prodrug strategy
[Carrier linked prodrug]
|
|||||
| Improved property |
Achieve targeted delivery to eyes
|
[2] | ||||
| Description |
Latanoprost is better absorbed than the parent compound through the cornea, and peak concentration of the active drug is in the aqueous humor 1-2 hours after topical dosing (15-30 ng/mL).
|
[3] | ||||
| Formula |
C26H40O5
|
|||||
| Canonical SMILES |
CC(C)OC(=O)CCCC=CCC1C(CC(C1CCC(CCC2=CC=CC=C2)O)O)O
|
|||||
| InChI |
1S/C26H40O5/c1-19(2)31-26(30)13-9-4-3-8-12-22-23(25(29)18-24(22)28)17-16-21(27)15-14-20-10-6-5-7-11-20/h3,5-8,10-11,19,21-25,27-29H,4,9,12-18H2,1-2H3/b8-3-/t21-,22+,23+,24-,25+/m0/s1
|
|||||
| InChIKey |
GGXICVAJURFBLW-CEYXHVGTSA-N
|
|||||
| CAS Number |
CAS 130209-82-4
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:6384
|
|||||
| Parent Drug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Parent Drug ID |
DJ03BX
|
|||||
| Parent Drug Name |
Latanoprost acid
|
|||||
| Synonyms |
Phxa 85; 41639-83-2; CHEBI:63925; UNII-EJ85341990; latanoprost free acid; Latanprost Free Acid; Phxa-85; EJ85341990; Latanoprost (free acid); PHXA85; Latonoprost acid; latanoprost-free acid; CHEMBL1050; latanoprost (free acid form); Latanoprost Related Compound E; GTPL1960; SCHEMBL1313159; DTXSID6040531; HMS3648J04; Latanoprost acid, >=98% (HPLC); BDBM50485606; MFCD08056084; ZINC13589951; HY-113756A; NCGC00165820-01; AS-56760; SC-44791; CS-0068357; Z6154; 639L832; SR-01000946512; SR-01000946512-1; Q27078867; Latanoprost Related Compound E, United States Pharmacopeia (USP) Reference Standard
Click to Show/Hide
|
|||||
| Formula |
C23H34O5
|
|||||
| Canonical SMILES |
C1C(C(C(C1O)CC=CCCCC(=O)O)CCC(CCC2=CC=CC=C2)O)O
|
|||||
| InChI |
1S/C23H34O5/c24-18(13-12-17-8-4-3-5-9-17)14-15-20-19(21(25)16-22(20)26)10-6-1-2-7-11-23(27)28/h1,3-6,8-9,18-22,24-26H,2,7,10-16H2,(H,27,28)/b6-1-/t18-,19+,20+,21-,22+/m0/s1
|
|||||
| InChIKey |
HNPFPERDNWXAGS-NFVOFSAMSA-N
|
|||||
| CAS Number |
CAS 41639-83-2
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:63925
|
|||||
| Target and Pathway | Top | |||||
|---|---|---|---|---|---|---|
| Target(s) | Prostaglandin F2-alpha receptor (PTGFR) | Target Info | Agonist | [1] | ||
| KEGG Pathway | Calcium signaling pathway | |||||
| Neuroactive ligand-receptor interaction | ||||||
| Reactome | Prostanoid ligand receptors | |||||
| G alpha (q) signalling events | ||||||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||||
| GPCRs, Class A Rhodopsin-like | ||||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||||
| Small Ligand GPCRs | ||||||
| GPCR ligand binding | ||||||
| GPCR downstream signaling | ||||||
| GPCRs, Other | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2014 | |||||
| REF 2 | Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008 Mar;7(3):255-70. | |||||
| REF 3 | Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014 Sep 26;8:1967-85. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

