Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T06671
(Former ID: TTDC00221)
|
|||||
| Target Name |
Interleukin-18 (IL18)
|
|||||
| Synonyms |
Interleukin-1 gamma; Interferon-gamma inducing factor; Interferon gamma-inducing factor; Iboctadekin; IL1F4; IL-18; IL-1 gamma; IGIF; IFN-gamma-inducing factor
Click to Show/Hide
|
|||||
| Gene Name |
IL18
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Adaptive immunity immunodeficiency [ICD-11: 4A01] | |||||
| Function |
Upon binding to IL18R1 and IL18RAP, forms a signaling ternary complex which activates NF-kappa-B, triggering synthesis of inflammatory mediators. Synergizes with IL12/interleukin-12 to induce IFNG synthesis from T-helper 1 (Th1) cells and natural killer (NK) cells. A proinflammatory cytokine primarily involved in polarized T-helper 1 (Th1) cell and natural killer (NK) cell immune responses.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MAAEPVEDNCINFVAMKFIDNTLYFIAEDDENLESDYFGKLESKLSVIRNLNDQVLFIDQ
GNRPLFEDMTDSDCRDNAPRTIFIISMYKDSQPRGMAVTISVKCEKISTLSCENKIISFK EMNPPDNIKDTKSDIIFFQRSVPGHDNKMQFESSSYEGYFLACEKERDLFKLILKKEDEL GDRSIMFTVQNED Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T63EMC | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | Tadekinig alfa | Drug Info | Phase 3 | XIAP deficiency | [2] | |
| 2 | GSK-1070806 | Drug Info | Phase 2 | Type-2 diabetes | [3] | |
| 3 | Iboctadekin | Drug Info | Phase 1 | Ovarian cancer | [4] | |
| 4 | Iboctadekin + Doxil | Drug Info | Phase 1 | Ovarian cancer | [5], [6] | |
| 5 | Iboctadekin + rituximab | Drug Info | Phase 1 | Follicular lymphoma | [7] | |
| 6 | MEDI-2338 | Drug Info | Phase 1 | Chronic obstructive pulmonary disease | [8] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | IL-18BP | Drug Info | Discontinued in Phase 1 | Rheumatoid arthritis | [9] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | Tadekinig alfa | Drug Info | [10] | |||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | Iboctadekin | Drug Info | [11] | |||
| 2 | Iboctadekin + Doxil | Drug Info | [12] | |||
| 3 | Iboctadekin + rituximab | Drug Info | [12] | |||
| 4 | MEDI-2338 | Drug Info | [13] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | IL-18BP | Drug Info | [14] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Chaps | Ligand Info | |||||
| Structure Description | Crystal structure of human interleukin-18 | PDB:3WO2 | ||||
| Method | X-ray diffraction | Resolution | 2.33 Å | Mutation | No | [15] |
| PDB Sequence |
YFGKLESKLS
10 VIRNLNDQVL20 FIDQGNRPLF30 EDMTDSDCRD40 NAPRTIFIIS50 MYKDSQPRGM 60 AVTISVKCEK70 ISTLSCENKI80 ISFKEMNPPD90 NIKDTKSDII100 FFQRSVPGHD 110 NKMQFESSSY120 EGYFLACEKE130 RDLFKLILKK140 EDELGDRSIM150 FTVQNED |
|||||
|
|
LYS4
3.698
SER7
3.383
LYS8
4.595
LEU9
3.804
SER10
4.369
VAL11
3.874
ARG13
4.359
ASP35
3.508
CYS38
3.739
ARG39
4.024
ASP40
4.978
ALA42
3.913
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
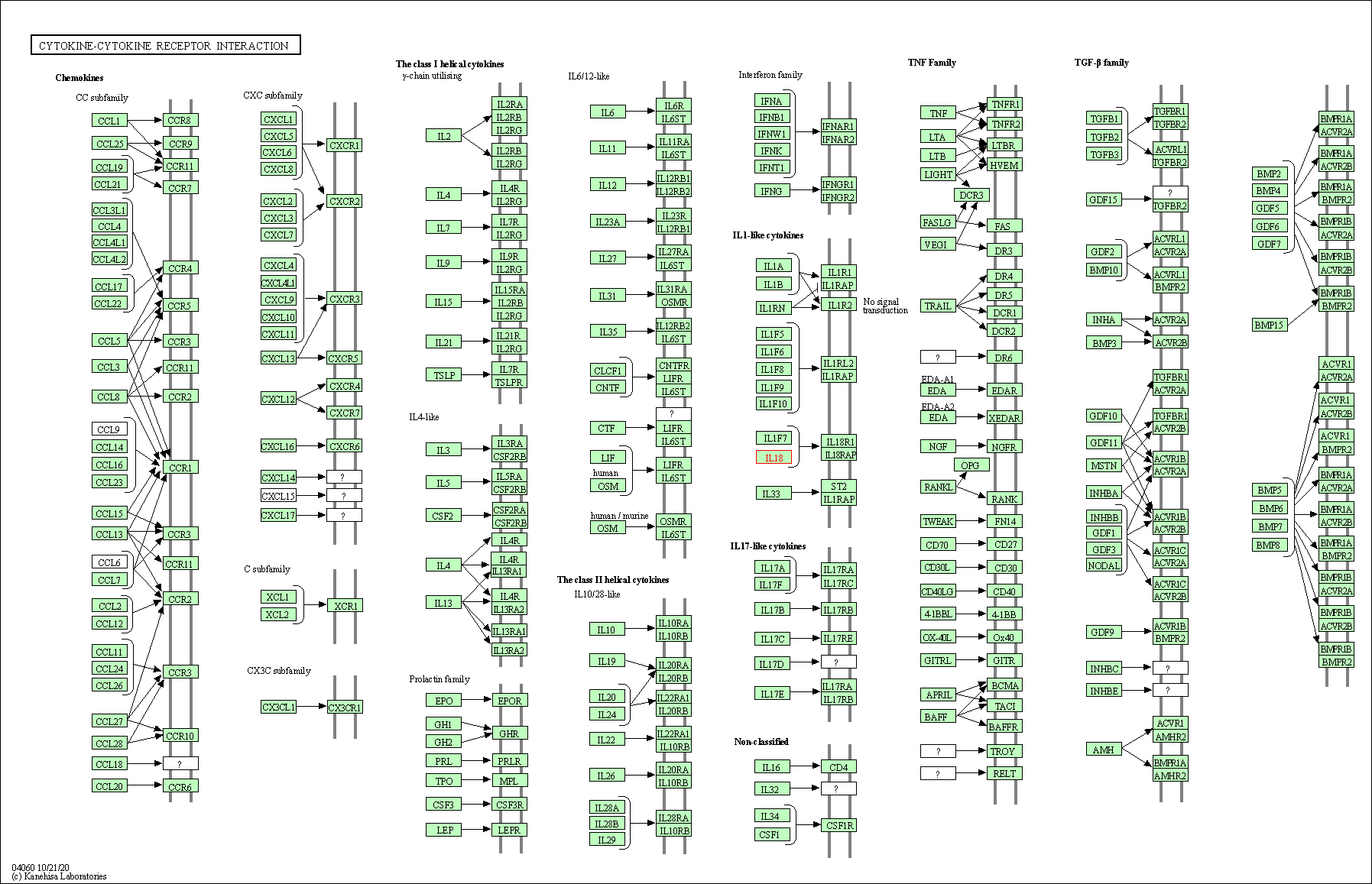

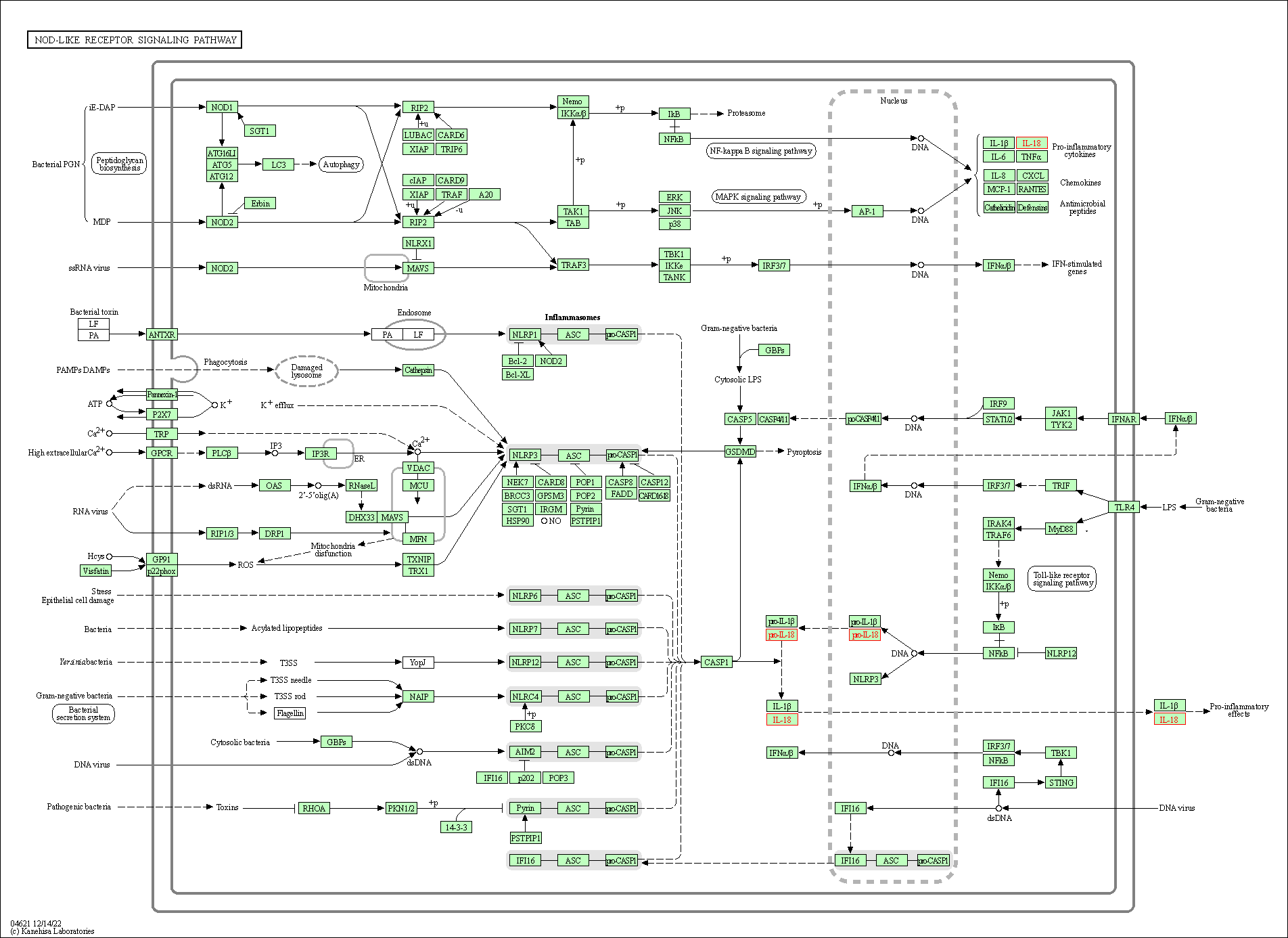
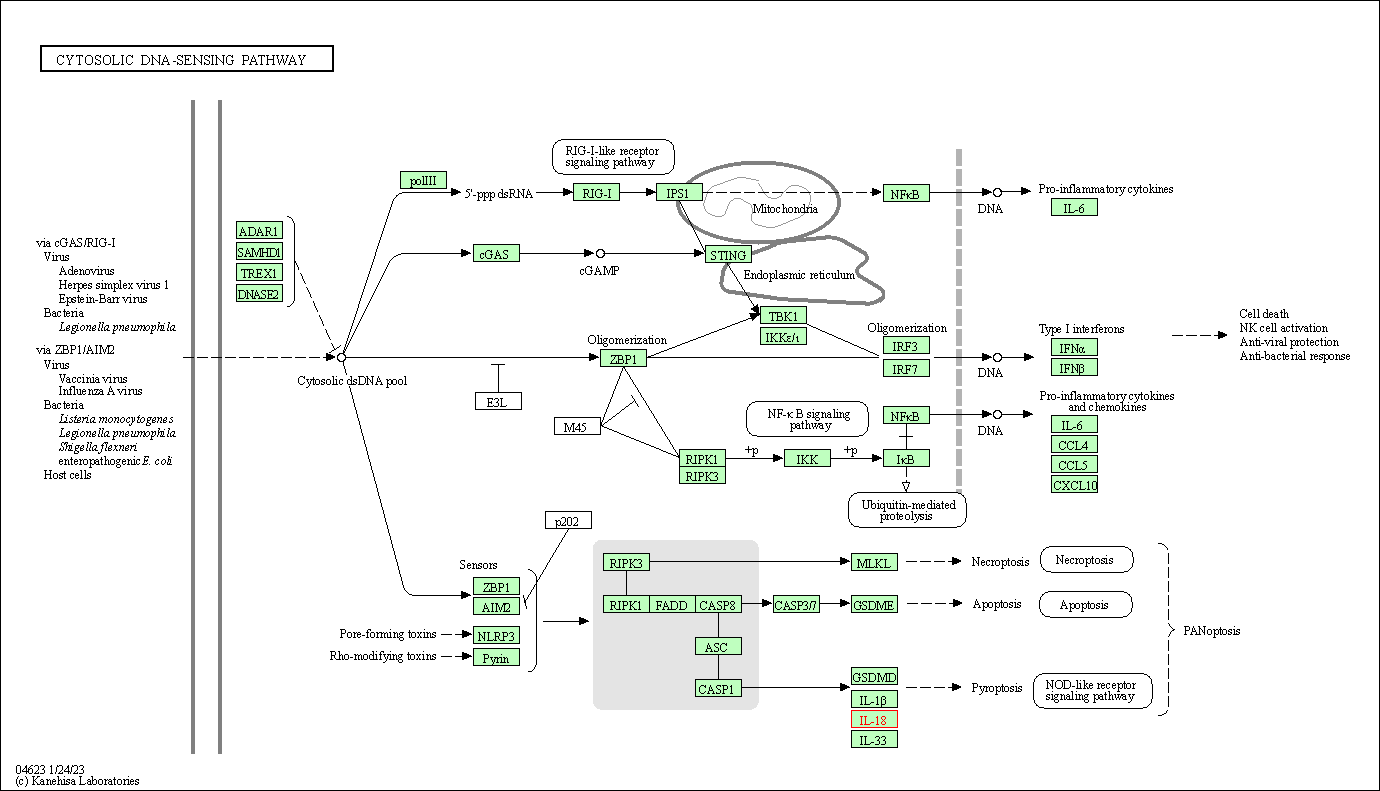
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NOD-like receptor signaling pathway | hsa04621 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Cytosolic DNA-sensing pathway | hsa04623 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 28 | Degree centrality | 3.01E-03 | Betweenness centrality | 5.75E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.20E-01 | Radiality | 1.39E+01 | Clustering coefficient | 3.47E-01 |
| Neighborhood connectivity | 2.31E+01 | Topological coefficient | 1.03E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 11 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | NOD-like receptor signaling pathway | |||||
| 3 | Cytosolic DNA-sensing pathway | |||||
| 4 | Salmonella infection | |||||
| 5 | Legionellosis | |||||
| 6 | African trypanosomiasis | |||||
| 7 | Malaria | |||||
| 8 | Tuberculosis | |||||
| 9 | Influenza A | |||||
| 10 | Inflammatory bowel disease (IBD) | |||||
| 11 | Rheumatoid arthritis | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| Panther Pathway | [+] 2 Panther Pathways | + | ||||
| 1 | Interleukin signaling pathway | |||||
| 2 | Toll receptor signaling pathway | |||||
| PID Pathway | [+] 5 PID Pathways | + | ||||
| 1 | IL27-mediated signaling events | |||||
| 2 | IL12-mediated signaling events | |||||
| 3 | IL23-mediated signaling events | |||||
| 4 | Cellular roles of Anthrax toxin | |||||
| 5 | IL12 signaling mediated by STAT4 | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Interleukin-1 processing | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Hypertrophy Model | |||||
| 2 | IL1 and megakaryotyces in obesity | |||||
| 3 | Corticotropin-releasing hormone | |||||
| 4 | NOD pathway | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010 November; 11(11): 1211-1220. | |||||
| REF 2 | ClinicalTrials.gov (NCT03512314) Therapeutic Use of Tadekinig Alfa in NLRC4 Mutation and XIAP Deficiency as Open Label Extension. U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT01648153) Investigate the Efficacy and Safety of GSK1070806 in Obese Subjects With T2DM. U.S. National Institutes of Health. | |||||
| REF 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008435) | |||||
| REF 5 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | |||||
| REF 6 | ClinicalTrials.gov (NCT00659178) Combination Study Of SB-485232 (Interleukin 18) And Doxil For Advanced Stage Epithelial Ovarian Cancer. U.S. National Institutes of Health. | |||||
| REF 7 | J Clin Oncol 27:15s, 2009 (suppl, abstr 8566). | |||||
| REF 8 | ClinicalTrials.gov (NCT01322594) A Study to Evaluate the Safety of MEDI2338 in Subjects With Chronic Obstructive Pulmonary Disease. U.S. National Institutes of Health. | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013227) | |||||
| REF 10 | Clinical pipeline report, company report or official report of AB2 Bio. | |||||
| REF 11 | Chemoimmunotherapy using pegylated liposomal Doxorubicin and interleukin-18 in recurrent ovarian cancer: a phase I dose-escalation study. Cancer Immunol Res. 2013 Sep;1(3):168-78. | |||||
| REF 12 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 13 | Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014; 9: 397-412. | |||||
| REF 14 | Emerging drugs for rheumatoid arthritis. Expert Opin Emerg Drugs. 2008 Mar;13(1):175-96. | |||||
| REF 15 | The structural basis for receptor recognition of human interleukin-18. Nat Commun. 2014 Dec 15;5:5340. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

