Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T15000
(Former ID: TTDC00259)
|
|||||
| Target Name |
Cytotoxic T-lymphocyte protein 4 (CTLA-4)
|
|||||
| Synonyms |
Cytotoxic T-lymphocyte-associated antigen 4; CTLA-4; CD152
Click to Show/Hide
|
|||||
| Gene Name |
CTLA4
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Liver cancer [ICD-11: 2C12] | |||||
| 2 | Melanoma [ICD-11: 2C30] | |||||
| Function |
Inhibitory receptor acting as a major negative regulator of T-cell responses. The affinity of CTLA4 for its natural B7 family ligands, CD80 and CD86, is considerably stronger than the affinity of their cognate stimulatory coreceptor CD28.
Click to Show/Hide
|
|||||
| BioChemical Class |
Immunoglobulin
|
|||||
| UniProt ID | ||||||
| Sequence |
MACLGFQRHKAQLNLATRTWPCTLLFFLLFIPVFCKAMHVAQPAVVLASSRGIASFVCEY
ASPGKATEVRVTVLRQADSQVTEVCAATYMMGNELTFLDDSICTGTSSGNQVNLTIQGLR AMDTGLYICKVELMYPPPYYLGIGNGTQIYVIDPEPCPDSDFLLWILAAVSSGLFFYSFL LTAVSLSKMLKKRSPLTTGVYVKMPPTEPECEKQFQPYFIPIN Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T75U40 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Ipilimumab | Drug Info | Approved | Melanoma | [1], [2], [3] | |
| 2 | Tremelimumab | Drug Info | Approved | Hepatocellular carcinoma | [4] | |
| Clinical Trial Drug(s) | [+] 22 Clinical Trial Drugs | + | ||||
| 1 | AK04 | Drug Info | Phase 3 | Actinic keratosis | [5] | |
| 2 | KN046 | Drug Info | Phase 3 | Non-small-cell lung cancer | [6] | |
| 3 | AK104 | Drug Info | Phase 2 | Cervical cancer | [7] | |
| 4 | Lorigerlimab | Drug Info | Phase 2 | Prostate cancer | [8] | |
| 5 | Vudalimab | Drug Info | Phase 2 | Prostate cancer | [9] | |
| 6 | AGEN1884 | Drug Info | Phase 1/2 | Cervical cancer | [10] | |
| 7 | BMS-986288 | Drug Info | Phase 1/2 | Solid tumour/cancer | [11] | |
| 8 | MK-1308 | Drug Info | Phase 1/2 | Solid tumour/cancer | [12] | |
| 9 | Zalifrelimab | Drug Info | Phase 1/2 | Cervical cancer | [13] | |
| 10 | A337 | Drug Info | Phase 1 | Aggressive cancer | [14] | |
| 11 | ADG116 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| 12 | AGEN1181 | Drug Info | Phase 1 | Solid tumour/cancer | [16] | |
| 13 | ALPN-202 | Drug Info | Phase 1 | Solid tumour/cancer | [17] | |
| 14 | BMS-986249 | Drug Info | Phase 1 | Solid tumour/cancer | [10] | |
| 15 | MEDI5752 | Drug Info | Phase 1 | Solid tumour/cancer | [18] | |
| 16 | MGD019 | Drug Info | Phase 1 | Solid tumour/cancer | [19] | |
| 17 | ONC-392 | Drug Info | Phase 1 | Non-small-cell lung cancer | [20] | |
| 18 | SI-B003 | Drug Info | Phase 1 | Solid tumour/cancer | [21] | |
| 19 | TRemelimumab + MEDI0562 | Drug Info | Phase 1 | Solid tumour/cancer | [22] | |
| 20 | XmAb20717 | Drug Info | Phase 1 | Solid tumour/cancer | [23] | |
| 21 | XmAb22841 | Drug Info | Phase 1 | Solid tumour/cancer | [24] | |
| 22 | YH001 | Drug Info | Phase 1 | Aggressive cancer | [25] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | Tremelimumab | Drug Info | [26] | |||
| 2 | Concatameric CTLA4Ig | Drug Info | [26] | |||
| 3 | CTLA-4-XTEN | Drug Info | [26] | |||
| Inhibitor | [+] 12 Inhibitor drugs | + | ||||
| 1 | KN046 | Drug Info | [28] | |||
| 2 | AK104 | Drug Info | [29] | |||
| 3 | BMS-986288 | Drug Info | [32] | |||
| 4 | MK-1308 | Drug Info | [33] | |||
| 5 | ADG116 | Drug Info | [35] | |||
| 6 | AGEN1181 | Drug Info | [36] | |||
| 7 | ALPN-202 | Drug Info | [37] | |||
| 8 | MEDI5752 | Drug Info | [38] | |||
| 9 | MGD019 | Drug Info | [39] | |||
| 10 | ONC-392 | Drug Info | [40] | |||
| 11 | Alpha-D-Mannose | Drug Info | [45] | |||
| 12 | Fucose | Drug Info | [45] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | AGEN1884 | Drug Info | [10], [22] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | TRemelimumab + MEDI0562 | Drug Info | [22] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| T-cell-specific surface glycoprotein CD28 (CD28) | 31.250 (60/192) | 1.97E-13 |
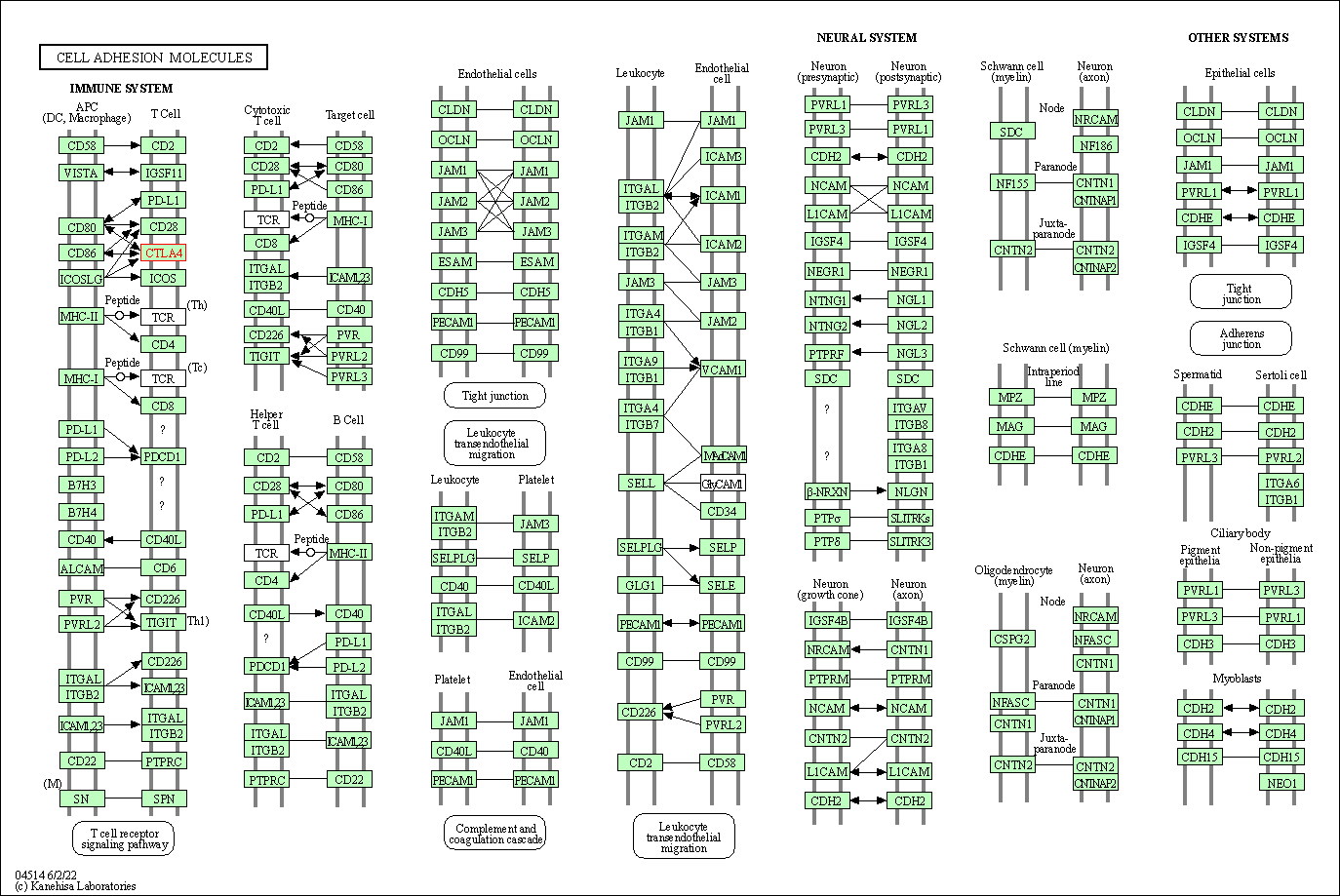
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cell adhesion molecules | hsa04514 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
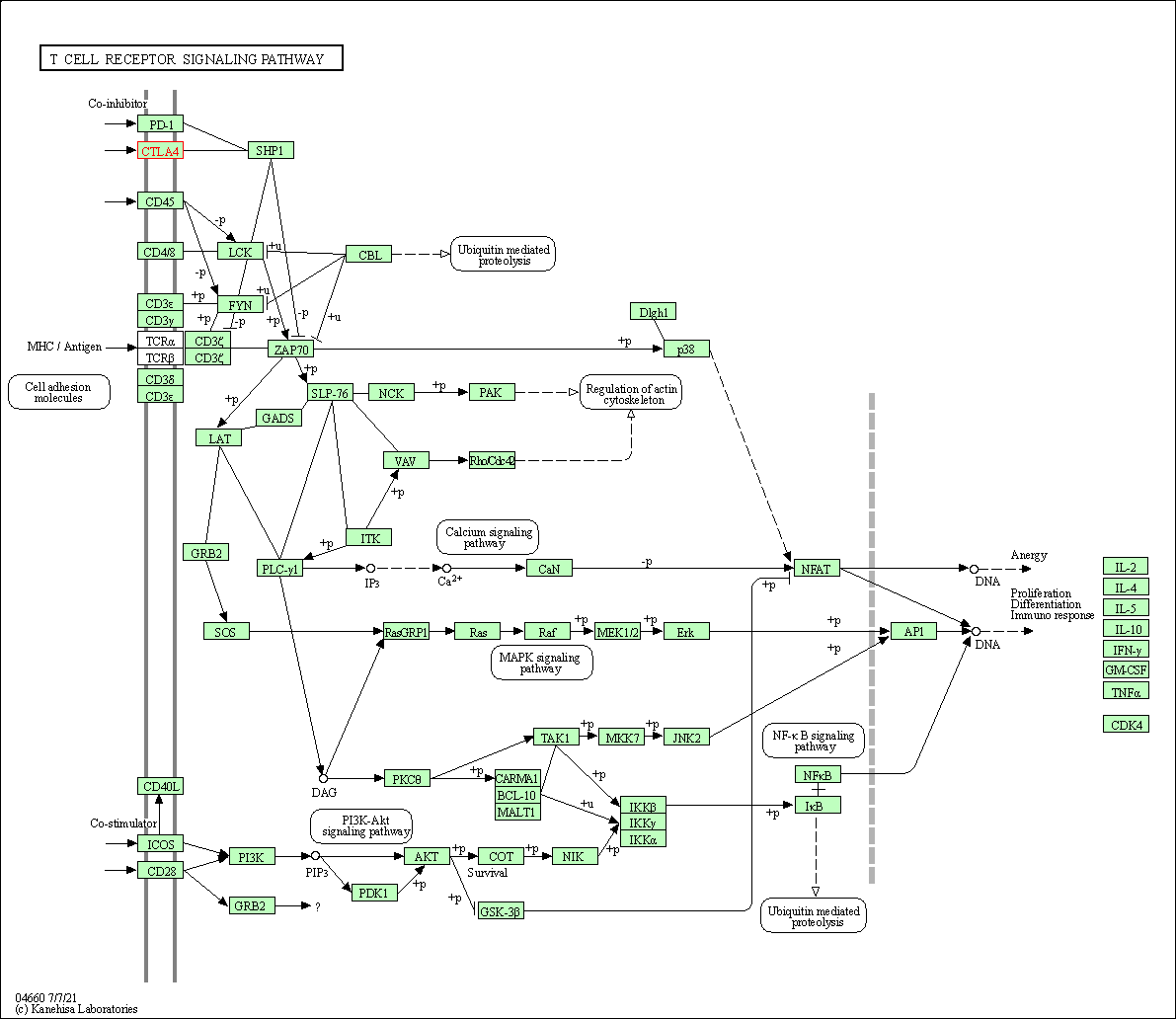
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 15 | Degree centrality | 1.61E-03 | Betweenness centrality | 5.19E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.28E-01 | Radiality | 1.40E+01 | Clustering coefficient | 1.33E-01 |
| Neighborhood connectivity | 3.35E+01 | Topological coefficient | 1.06E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Cell adhesion molecules (CAMs) | |||||
| 2 | T cell receptor signaling pathway | |||||
| 3 | Autoimmune thyroid disease | |||||
| 4 | Rheumatoid arthritis | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Calcineurin-regulated NFAT-dependent transcription in lymphocytes | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | CTLA4 inhibitory signaling | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Vitamin D Receptor Pathway | |||||
| 2 | T-Cell Receptor and Co-stimulatory Signaling | |||||
| 3 | Allograft Rejection | |||||
| 4 | Costimulation by the CD28 family | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Mullard A: 2010 FDA drug approvals. Nat Rev Drug Discov. 2011 Feb;10(2):82-5. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6888). | |||||
| REF 3 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 4 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761289. | |||||
| REF 5 | ClinicalTrials.gov (NCT00308867) Photodynamic Therapy With PD P 506 A or Its Placebo Compared With Cryosurgery for the Treatment of Mild to Moderate Actinic Keratosis. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT04474119) KN046 in Subjects With Advanced Squamous Non-small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT04380805) A Study of AK104, a PD-1/CTLA-4 Bispecific Antibody in Subjects With Recurrent/Metastatic Cervical Cancer. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT05848011) A Phase 2, Randomized, Open-Label, Study of Lorigerlimab With Docetaxel or Docetaxel Alone in Participants With Metastatic Castration-Resistant Prostate Cancer. U.S.National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT05005728) Phase 2 Multiple-Dose, Multiple-Arm, Parallel Assignment Study to Evaluate the Safety, Tolerability, and Preliminary Efficacy of XmAb?20717 Alone or in Combination With Chemotherapy or Targeted Therapies in Selected Subjects With Metastatic Castration-Resistant Prostate Cancer. U.S.National Institutes of Health. | |||||
| REF 10 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 11 | ClinicalTrials.gov (NCT03994601) An Investigational Immunotherapy Study of BMS-986288 Alone and in Combination With Nivolumab in Advanced Solid Cancers. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT03179436) Study of Quavonlimab (MK-1308) in Combination With Pembrolizumab (MK-3475) in Advanced Solid Tumors (MK-1308-001). U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT05033132) An Open-label, Multicenter Phase II Study Evaluating Balstilimab Alone or Balstilimab in Combination With Zalifrelimab in Patients With Advanced Cervical Cancer. U.S.National Institutes of Health. | |||||
| REF 14 | Clinical pipeline report, company report or official report of Klus Pharma | |||||
| REF 15 | ClinicalTrials.gov (NCT04501276) ADG116 in Patients With Advanced/Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT03860272) Fc-Engineered Anti-CTLA-4 Monoclonal Antibody in Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT04186637) An Open-label Study of ALPN-202 in Subjects With Advanced Malignancies (NEON-1). U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT03530397) A Study to Evaluate MEDI5752 in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT03761017) MGD019 DART Protein in Unresectable/Metastatic Cancer. U.S. National Institutes of Health. | |||||
| REF 20 | ClinicalTrials.gov (NCT04140526) Safety, PK and Efficacy of ONC-392 in Monotherapy and in Combination of Anti-PD-1 in Advanced Solid Tumors and NSCLC (PRESERVE-001). U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT04606472) A Study of SI-B003, a PD-1/CTLA-4 Bispecific Antibody, in Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 22 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 23 | Development of Inhibitors of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Signaling Pathway.J Med Chem. 2019 Feb 28;62(4):1715-1730. | |||||
| REF 24 | ClinicalTrials.gov (NCT03849469) A Study of XmAb22841 Monotherapy & in Combination w/ Pembrolizumab in Subjects w/ Selected Advanced Solid Tumors (DUET-4). U.S. National Institutes of Health. | |||||
| REF 25 | ClinicalTrials.gov (NCT04699929) A Open-Label, Phase I Dose Escalation Study to Evaluate the Safety, Tolerability,Efficacy and Pharmacokinetics of YH001 in Subjects With Advanced Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 26 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2743). | |||||
| REF 27 | A multicenter, open-label phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) monotherapy in previously treated advanced non-small-cell lung cancer (AK104-202 study). Lung Cancer. 2023 Oct;184:107355. | |||||
| REF 28 | Clinical pipeline report, company report or official report of Alphamab Oncology. | |||||
| REF 29 | Clinical pipeline report, company report or official report of Akeso Biopharma. | |||||
| REF 30 | Clinical pipeline report, company report or official report of MacroGenics | |||||
| REF 31 | Clinical pipeline report, company report or official report of Xencor | |||||
| REF 32 | Clinical pipeline report, company report or official report of CytomX Therapeutics. | |||||
| REF 33 | Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol. 2021 Mar;32(3):395-403. | |||||
| REF 34 | Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J Clin Oncol. 2022 Mar 1;40(7):762-771. | |||||
| REF 35 | Clinical pipeline report, company report or official report of Adagene. | |||||
| REF 36 | Clinical pipeline report, company report or official report of Agenus. | |||||
| REF 37 | Clinical pipeline report, company report or official report of Alpine Immune Sciences. | |||||
| REF 38 | Bispecific Antibodies to PD-1 and CTLA4: Doubling Down on T Cells to Decouple Efficacy from Toxicity. Cancer Discov. 2021 May;11(5):1008-1010. | |||||
| REF 39 | Development and Preliminary Clinical Activity of PD-1-Guided CTLA-4 Blocking Bispecific DART Molecule. Cell Rep Med. 2020 Dec 22;1(9):100163. | |||||
| REF 40 | Clinical pipeline report, company report or official report of OncoImmune. | |||||
| REF 41 | Clinical pipeline report, company report or official report of SystImmune. | |||||
| REF 42 | Clinical pipeline report, company report or official report of Xencor. | |||||
| REF 43 | Clinical pipeline report, company report or official report of Xencor. | |||||
| REF 44 | Clinical pipeline report, company report or official report of Biocytogen | |||||
| REF 45 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 46 | Therapeutic applications of aptamers. Expert Opin Investig Drugs. 2008 Jan;17(1):43-60. | |||||
| REF 47 | Clinical pipeline report, company report or official report of Biocytogen | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

