Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T15334
(Former ID: TTDC00124)
|
|||||
| Target Name |
Cholesteryl ester transfer protein (CETP)
|
|||||
| Synonyms |
Lipid transfer protein I; Cholesterol ester transfer protein
Click to Show/Hide
|
|||||
| Gene Name |
CETP
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Arterial occlusive disease [ICD-11: BD40] | |||||
| 2 | Cardiovascular disease [ICD-11: BA00-BE2Z] | |||||
| 3 | Hyper-lipoproteinaemia [ICD-11: 5C80] | |||||
| 4 | Myocardial infarction [ICD-11: BA41-BA43] | |||||
| Function |
Allows the net movement of cholesteryl ester from high density lipoproteins/HDL to triglyceride-rich very low density lipoproteins/VLDL, and the equimolar transport of triglyceride from VLDL to HDL. Regulates the reverse cholesterol transport, by which excess cholesterol is removed from peripheral tissues and returned to the liver for elimination. Involved in the transfer of neutral lipids, including cholesteryl ester and triglyceride, among lipoprotein particles.
Click to Show/Hide
|
|||||
| BioChemical Class |
Bactericidal permeability increasing protein
|
|||||
| UniProt ID | ||||||
| Sequence |
MLAATVLTLALLGNAHACSKGTSHEAGIVCRITKPALLVLNHETAKVIQTAFQRASYPDI
TGEKAMMLLGQVKYGLHNIQISHLSIASSQVELVEAKSIDVSIQNVSVVFKGTLKYGYTT AWWLGIDQSIDFEIDSAIDLQINTQLTCDSGRVRTDAPDCYLSFHKLLLHLQGEREPGWI KQLFTNFISFTLKLVLKGQICKEINVISNIMADFVQTRAASILSDGDIGVDISLTGDPVI TASYLESHHKGHFIYKNVSEDLPLPTFSPTLLGDSRMLYFWFSERVFHSLAKVAFQDGRL MLSLMGDEFKAVLETWGFNTNQEIFQEVVGGFPSQAQVTVHCLKMPKISCQNKGVVVNSS VMVKFLFPRPDQQHSVAYTFEEDIVTTVQASYSKKKLFLSLLDFQITPKTVSNLTESSSE SVQSFLQSMITAVGIPEVMSRLEVVFTALMNSKGVSLFDIINPEIITRDGFLLLQMDFGF PEHLLVDFLQSLS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T48UT8 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 7 Clinical Trial Drugs | + | ||||
| 1 | Anacetrapib | Drug Info | Phase 3 | Arteriosclerosis | [2], [3] | |
| 2 | Dalcetrapib | Drug Info | Phase 3 | Hyperlipidaemia | [4] | |
| 3 | Evacetrapib | Drug Info | Phase 3 | Cardiovascular disease | [5], [6] | |
| 4 | DRL-17822 | Drug Info | Phase 2 | Arteriosclerosis | [7] | |
| 5 | JTT-302 | Drug Info | Phase 2 | Lipid metabolism disorder | [8] | |
| 6 | TA-8995 | Drug Info | Phase 2 | Hyperlipidaemia | [9] | |
| 7 | BAY-60-5521 | Drug Info | Phase 1 | Arteriosclerosis | [10] | |
| Discontinued Drug(s) | [+] 6 Discontinued Drugs | + | ||||
| 1 | CETi-1 | Drug Info | Discontinued in Phase 2 | Arteriosclerosis | [11] | |
| 2 | Torcetrapib | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [12] | |
| 3 | CP-800569 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [13] | |
| 4 | DS-1442 | Drug Info | Discontinued in Phase 1 | Dyslipidemia | [14] | |
| 5 | PF-3185043 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [15] | |
| 6 | R7232 | Drug Info | Discontinued in Phase 1 | Dyslipidemia | [16] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | BAY-38-1315 | Drug Info | Preclinical | Arteriosclerosis | [17] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 17 Inhibitor drugs | + | ||||
| 1 | Anacetrapib | Drug Info | [18], [19], [20] | |||
| 2 | Dalcetrapib | Drug Info | [1] | |||
| 3 | Evacetrapib | Drug Info | [21] | |||
| 4 | DRL-17822 | Drug Info | [21] | |||
| 5 | JTT-302 | Drug Info | [21] | |||
| 6 | TA-8995 | Drug Info | [22] | |||
| 7 | BAY-60-5521 | Drug Info | [23] | |||
| 8 | Torcetrapib | Drug Info | [20], [25], [26] | |||
| 9 | CP-800569 | Drug Info | [27], [28] | |||
| 10 | PF-3185043 | Drug Info | [27], [28] | |||
| 11 | R7232 | Drug Info | [1], [30] | |||
| 12 | BAY-38-1315 | Drug Info | [31] | |||
| 13 | NSC-40331 | Drug Info | [32] | |||
| 14 | NSC-89508 | Drug Info | [32] | |||
| 15 | SC-795 | Drug Info | [32] | |||
| 16 | TETRAHYDROQUINOLINE A | Drug Info | [33] | |||
| 17 | TETRAHYDROQUINOLINE B | Drug Info | [33] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | DS-1442 | Drug Info | [29] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Torcetrapib | Ligand Info | |||||
| Structure Description | Crystal structure of cholesteryl ester transfer protein in complex with inhibitors | PDB:4EWS | ||||
| Method | X-ray diffraction | Resolution | 2.59 Å | Mutation | Yes | [34] |
| PDB Sequence |
TSHEAGIVCR
14 ITKPALLVLN24 HETAKVIQTA34 FQRASYPDIT44 GEKAMMLLGQ54 VKYGLHNIQI 64 SHLSIASSQV74 ELVEAKSIDV84 SIQDVSVVFK94 GTLKYGYTTA104 WWLGIDQSID 114 FEIDSAIDLQ124 INTQLTADSG134 RVRTDAPDCY144 LSFHKLLLHL154 QGEREPGWIK 164 QLFTNFISFT174 LKLVLKGQIC184 KEINVISNIM194 ADFVQTRAAS204 ILSDGDIGVD 214 ISLTGDPVIT224 ASYLESHHKG234 HFIYKDVSED244 LPLPTFSPTL254 LGDSRMLYFW 264 FSERVFHSLA274 KVAFQDGRLM284 LSLMGDEFKA294 VLETWGFNTN304 QEIFQEVVGG 314 FPSQAQVTVH324 CLKMPKISCQ334 NKGVVVDSSV344 MVKFLFPRPD354 QQHSVAYTFE 364 EDIVTTVQAS374 YSKKKLFLSL384 LDFQITPKTV394 SNLTESSSES404 IQSFLQSMIT 414 AVGIPEVMSR424 LEVVFTALMN434 SKGVSLFDII444 NPEIITRDGF454 LLLQMDFGFP 464 EHLLVDFLQS474 LS
|
|||||
|
|
ILE11
3.288
VAL12
4.366
CYS13
3.764
ILE15
3.374
LEU129
3.502
GLY134
4.916
VAL136
3.182
THR138
4.922
ALA195
3.319
VAL198
3.547
GLN199
3.235
ARG201
4.020
ALA202
3.330
|
|||||
| Ligand Name: Cholesteryl oleate | Ligand Info | |||||
| Structure Description | Crystal Structure of Cholesteryl Ester Transfer Protein | PDB:2OBD | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | Yes | [35] |
| PDB Sequence |
TSHEAGIVCR
14 ITKPALLVLN24 HETAKVIQTA34 FQRASYPDIT44 GEKAMMLLGQ54 VKYGLHNIQI 64 SHLSIASSQV74 ELVEAKSIDV84 SIQDVSVVFK94 GTLKYGYTTA104 WWLGIDQSID 114 FEIDSAIDLQ124 INTQLTADSG134 RVRTDAPDCY144 LSFHKLLLHL154 QGEREPGWIK 164 QLFTNFISFT174 LKLVLKGQIC184 KEINVISNIM194 ADFVQTRAAS204 ILSDGDIGVD 214 ISLTGDPVIT224 ASYLESHHKG234 HFIYKDVSED244 LPLPTFSPTL254 LGDSRMLYFW 264 FSERVFHSLA274 KVAFQDGRLM284 LSLMGDEFKA294 VLETWGFNTN304 QEIFQEVVGG 314 FPSQAQVTVH324 CLKMPKISCQ334 NKGVVVDSSV344 MVKFLFPRPD354 QQHSVAYTFE 364 EDIVTTVQAS374 YSKKKLFLSL384 LDFQITPKTV394 SNLTESSSES404 IQSFLQSMIT 414 AVGIPEVMSR424 LEVVFTALMN434 SKGVSLFDII444 NPEIITRDGF454 LLLQMDFGFP 464 EHLLVDFLQS474 LS
|
|||||
|
|
ILE11
3.722
CYS13
4.085
ILE15
3.573
LEU23
4.220
THR27
3.717
VAL30
4.007
ILE31
3.737
ILE82
3.727
VAL84
3.845
ILE86
4.880
ILE125
3.796
THR127
3.745
LEU129
4.708
VAL136
3.647
THR138
3.829
ILE187
4.527
SER191
3.847
ALA195
3.954
VAL198
3.473
GLN199
3.503
ALA202
3.580
ILE205
4.301
LEU206
3.905
ILE215
4.109
LEU228
3.562
SER230
3.632
HIS232
3.182
LEU261
3.552
PHE263
3.652
PHE265
3.917
PHE270
4.091
LEU285
4.894
LEU287
3.951
PHE292
3.284
VAL295
3.763
LEU296
3.832
VAL321
3.619
VAL323
3.462
ILE331
4.738
VAL338
4.055
VAL340
3.436
SER342
4.846
VAL344
4.375
VAL346
4.096
PHE348
4.454
ILE367
3.641
THR369
3.465
VAL371
3.969
ALA373
3.944
TYR375
3.638
LEU380
4.460
LEU382
3.772
MET412
4.029
ILE413
4.212
VAL416
3.610
GLY417
3.416
ILE418
3.891
VAL421
3.566
MET422
3.625
LEU425
3.523
PHE429
3.772
MET433
4.129
VAL438
3.803
PHE441
3.595
ILE443
4.096
PRO446
4.362
ILE448
3.985
LEU455
3.906
LEU457
3.438
MET459
3.948
PHE461
3.555
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
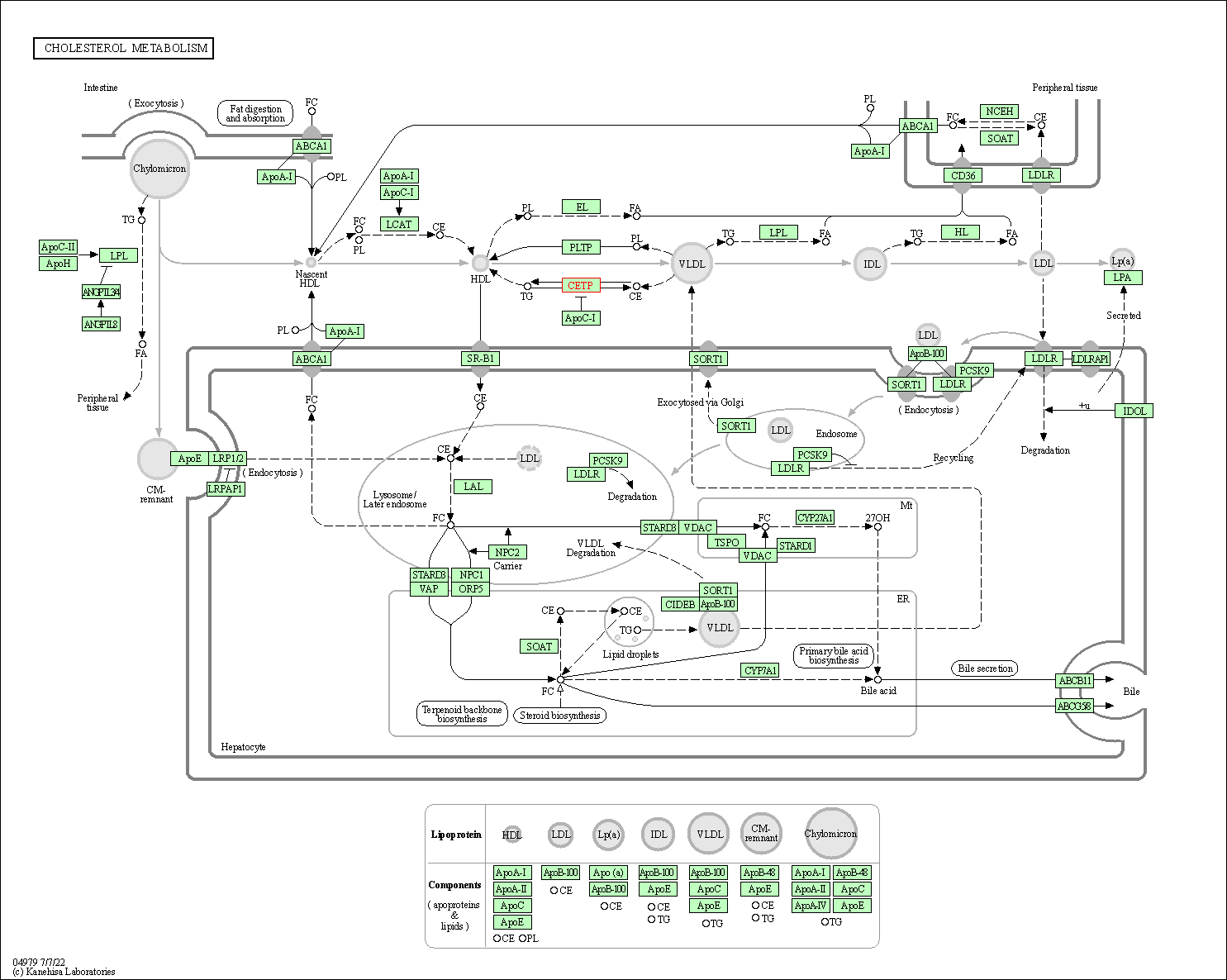
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cholesterol metabolism | hsa04979 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 4 | Degree centrality | 4.30E-04 | Betweenness centrality | 2.05E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.78E-01 | Radiality | 1.29E+01 | Clustering coefficient | 3.33E-01 |
| Neighborhood connectivity | 1.53E+01 | Topological coefficient | 3.41E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | CCKR signaling map ST | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | LDL-mediated lipid transport | |||||
| 2 | HDL-mediated lipid transport | |||||
| WikiPathways | [+] 2 WikiPathways | + | ||||
| 1 | Statin Pathway | |||||
| 2 | Lipid digestion, mobilization, and transport | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of Roche (2009). | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8400). | |||||
| REF 3 | Emerging antidyslipidemic drugs. Expert Opin Emerg Drugs. 2008 Jun;13(2):363-81. | |||||
| REF 4 | ClinicalTrials.gov (NCT00658515) A Study of RO4607381 in Stable Coronary Heart Disease Patients With Recent Acute Coronary Syndrome. U.S. National Institutes of Health. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8401). | |||||
| REF 6 | ClinicalTrials.gov (NCT02227784) A Study of Evacetrapib (LY2484595) in Participants With High Cholesterol. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT01388816) A Safety and Efficacy Study of DRL-17822, a Cholesteryl Ester Transfer Protein (CETP) Inhibitor, in Patients With Abnormal Cholesterol Levels. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT00748852) Safety Study of JTT-302 in Subjects With Low HDL-C Levels. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT01970215) TA-8995: Its Use in Patients With Mild Dyslipidaemia (TULIP). U.S. National Institutes of Health. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034993) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008445) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015157) | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025536) | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036983) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025537) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027351) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017234) | |||||
| REF 18 | Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein (C... Br J Clin Pharmacol. 2009 May;67(5):520-6. | |||||
| REF 19 | Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2009 Apr;31(3):183-227. | |||||
| REF 20 | The end of the road for CETP inhibitors after torcetrapib Curr Opin Cardiol. 2009 Jul;24(4):364-71. | |||||
| REF 21 | The dyslipidaemia market. Nat Rev Drug Discov. 2014 Nov;13(11):807-8. | |||||
| REF 22 | Amgen To Acquire Privately-Held Dezima Pharma | |||||
| REF 23 | Single dose pharmacokinetics, pharmacodynamics, tolerability and safety of BAY 60-5521, a potent inhibitor of cholesteryl ester transfer protein. Br J Clin Pharmacol. 2012 Feb;73(2):210-8. | |||||
| REF 24 | CETi-1. AVANT. Curr Opin Investig Drugs. 2004 Mar;5(3):334-8. | |||||
| REF 25 | Safety and tolerability of dalcetrapib. Am J Cardiol. 2009 Jul 1;104(1):82-91. | |||||
| REF 26 | A Translational Medicine perspective of the development of torcetrapib: Does the failure of torcetrapib development cast a shadow on future develop... Biochem Pharmacol. 2009 Aug 15;78(4):315-25. | |||||
| REF 27 | Pfizer. Product Development Pipeline. March 31 2009. | |||||
| REF 28 | Flavonoids as inhibitors of MRP1-like efflux activity in human erythrocytes. A structure-activity relationship study. Oncol Res. 2003;13(11):463-9. | |||||
| REF 29 | Ds-1442b is a Novel, Potent Cetp Inhibitor That Reduces Atherosclerosis by Hdl Elevation and Non-hdl Reduction. Circulation. 2012; 126: A11806. | |||||
| REF 30 | Phase 2 study of bosutinib, a Src inhibitor, in adults with recurrent glioblastoma. J Neurooncol. 2015 Feb;121(3):557-63. | |||||
| REF 31 | Chromanol derivatives--a novel class of CETP inhibitors. Bioorg Med Chem Lett. 2011 Jan 1;21(1):488-91. | |||||
| REF 32 | Discovery of new cholesteryl ester transfer protein inhibitors via ligand-based pharmacophore modeling and QSAR analysis followed by synthetic expl... Eur J Med Chem. 2010 Apr;45(4):1598-617. | |||||
| REF 33 | Design and synthesis of potent inhibitors of cholesteryl ester transfer protein (CETP) exploiting a 1,2,3,4-tetrahydroquinoline platform. Bioorg Med Chem Lett. 2009 May 1;19(9):2456-60. | |||||
| REF 34 | Crystal structures of cholesteryl ester transfer protein in complex with inhibitors. J Biol Chem. 2012 Oct 26;287(44):37321-9. | |||||
| REF 35 | Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol. 2007 Feb;14(2):106-13. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

