Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T64969
(Former ID: TTDR00576)
|
|||||
| Target Name |
Epidermal growth factor receptor variant III (EGFR vIII)
|
|||||
| Synonyms |
ERBB1 variant III; ERBB variant III
Click to Show/Hide
|
|||||
| Gene Name |
EGFR
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Brain cancer [ICD-11: 2A00] | |||||
| 2 | Liver cancer [ICD-11: 2C12] | |||||
| 3 | Lung cancer [ICD-11: 2C25] | |||||
| 4 | Pancreatic cancer [ICD-11: 2C10] | |||||
| Function |
Receptor tyrosine kinase binding ligands of the EGF family and activating several signaling cascades to convert extracellular cues into appropriate cellular responses. Known ligands include EGF, TGFA/TGF-alpha, AREG, epigen/EPGN, BTC/betacellulin, epiregulin/EREG and HBEGF/heparin-binding EGF. Ligand binding triggers receptor homo- and/or heterodimerization and autophosphorylation on key cytoplasmic residues. The phosphorylated receptor recruits adapter proteins like GRB2 which in turn activates complex downstream signaling cascades. Activates at least 4 major downstream signaling cascades including the RAS-RAF-MEK-ERK, PI3 kinase-AKT, PLCgamma-PKC and STATs modules. May also activate the NF-kappa-B signaling cascade. Also directly phosphorylates other proteins like RGS16, activating its GTPase activity and probably coupling the EGF receptor signaling to the G protein-coupled receptor signaling. Also phosphorylates MUC1 and increases its interaction with SRC and CTNNB1/beta-catenin. Plays a role in enhancing learning and memory performance (By similarity).
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MRPSGTAGAALLALLAALCPASRALEEKKVCQGTSNKLTQLGTFEDHFLSLQRMFNNCEV
VLGNLEITYVQRNYDLSFLKTIQEVAGYVLIALNTVERIPLENLQIIRGNMYYENSYALA VLSNYDANKTGLKELPMRNLQEILHGAVRFSNNPALCNVESIQWRDIVSSDFLSNMSMDF QNHLGSCQKCDPSCPNGSCWGAGEENCQKLTKIICAQQCSGRCRGKSPSDCCHNQCAAGC TGPRESDCLVCRKFRDEATCKDTCPPLMLYNPTTYQMDVNPEGKYSFGATCVKKCPRNYV VTDHGSCVRACGADSYEMEEDGVRKCKKCEGPCRKVCNGIGIGEFKDSLSINATNIKHFK NCTSISGDLHILPVAFRGDSFTHTPPLDPQELDILKTVKEITGFLLIQAWPENRTDLHAF ENLEIIRGRTKQHGQFSLAVVSLNITSLGLRSLKEISDGDVIISGNKNLCYANTINWKKL FGTSGQKTKIISNRGENSCKATGQVCHALCSPEGCWGPEPRDCVSCRNVSRGRECVDKCN LLEGEPREFVENSECIQCHPECLPQAMNITCTGRGPDNCIQCAHYIDGPHCVKTCPAGVM GENNTLVWKYADAGHVCHLCHPNCTYGCTGPGLEGCPTNGPKIPSIATGMVGALLLLLVV ALGIGLFMRRRHIVRKRTLRRLLQERELVEPLTPSGEAPNQALLRILKETEFKKIKVLGS GAFGTVYKGLWIPEGEKVKIPVAIKELREATSPKANKEILDEAYVMASVDNPHVCRLLGI CLTSTVQLITQLMPFGCLLDYVREHKDNIGSQYLLNWCVQIAKGMNYLEDRRLVHRDLAA RNVLVKTPQHVKITDFGLAKLLGAEEKEYHAEGGKVPIKWMALESILHRIYTHQSDVWSY GVTVWELMTFGSKPYDGIPASEISSILEKGERLPQPPICTIDVYMIMVKCWMIDADSRPK FRELIIEFSKMARDPQRYLVIQGDERMHLPSPTDSNFYRALMDEEDMDDVVDADEYLIPQ QGFFSSPSTSRTPLLSSLSATSNNSTVACIDRNGLQSCPIKEDSFLQRYSSDPTGALTED SIDDTFLPVPEYINQSVPKRPAGSVQNPVYHNQPLNPAPSRDPHYQDPHSTAVGNPEYLN TVQPTCVNSTFDSPAHWAQKGSHQISLDNPDYQQDFFPKEAKPNGIFKGSTAENAEYLRV APQSSEFIGA Click to Show/Hide
|
|||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 12 Clinical Trial Drugs | + | ||||
| 1 | Anti-EGFR V III CAR-T cells | Drug Info | Phase 1/2 | Glioma | [2] | |
| 2 | Anti-EGFRvIII CAR transduced PBL | Drug Info | Phase 1/2 | Brain cancer | [1] | |
| 3 | EGFRvIII CAR | Drug Info | Phase 1/2 | Glioblastoma multiforme | [1] | |
| 4 | AMG 596 | Drug Info | Phase 1 | Recurrent glioblastoma | [3], [4] | |
| 5 | Anti-EGFRvIII CAR T cells | Drug Info | Phase 1 | Glioblastoma multiforme | [5] | |
| 6 | CAR-T Cells targeting EGFRvIII | Drug Info | Phase 1 | Pancreatic cancer | [6] | |
| 7 | CAR-T cells targeting EGFRviii | Drug Info | Phase 1 | Glioblastoma multiforme | [7] | |
| 8 | CART-EGFRvIII T cells | Drug Info | Phase 1 | Recurrent glioblastoma | [8] | |
| 9 | D2C7 | Drug Info | Phase 1 | Glioblastoma of brain | [9] | |
| 10 | EGFRvIII CAR T cells | Drug Info | Phase 1 | Glioblastoma multiforme | [10] | |
| 11 | EGFRvIII-CARs | Drug Info | Phase 1 | Recurrent glioblastoma | [11] | |
| 12 | RG6156 | Drug Info | Phase 1 | Glioblastoma | [12] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| CAR-T-Cell-Therapy | [+] 8 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Anti-EGFR V III CAR-T cells | Drug Info | [2] | |||
| 2 | Anti-EGFRvIII CAR transduced PBL | Drug Info | [1] | |||
| 3 | Anti-EGFRvIII CAR T cells | Drug Info | [5] | |||
| 4 | CAR-T Cells targeting EGFRvIII | Drug Info | [6] | |||
| 5 | CAR-T cells targeting EGFRviii | Drug Info | [7] | |||
| 6 | CART-EGFRvIII T cells | Drug Info | [8], [15] | |||
| 7 | EGFRvIII CAR T cells | Drug Info | [10] | |||
| 8 | EGFRvIII-CARs | Drug Info | [11] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | EGFRvIII CAR | Drug Info | [13] | |||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | AMG 596 | Drug Info | [14] | |||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Pathway Affiliation
|
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| ErbB signaling pathway | hsa04012 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| FoxO signaling pathway | hsa04068 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
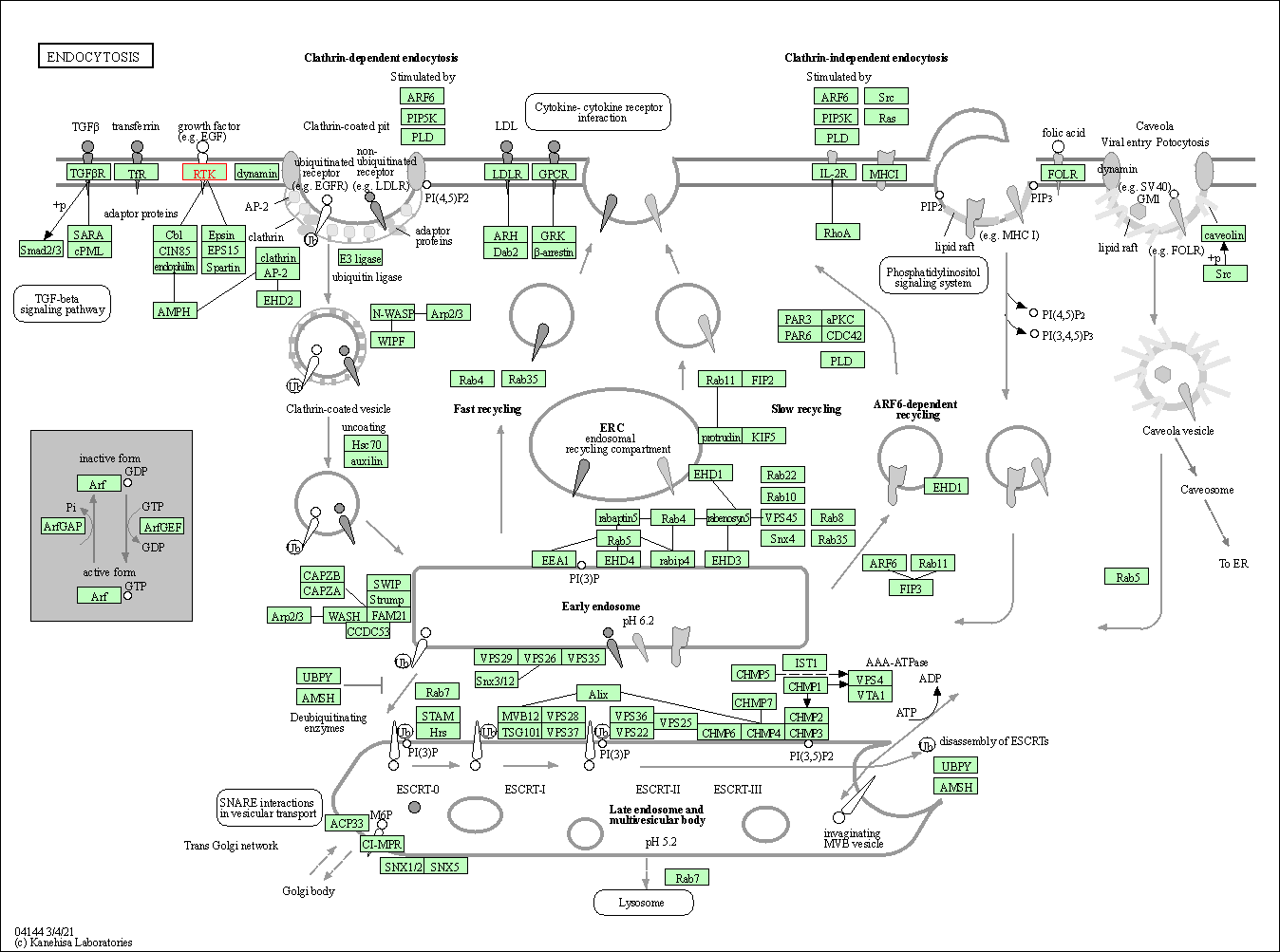
| Endocytosis | hsa04144 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
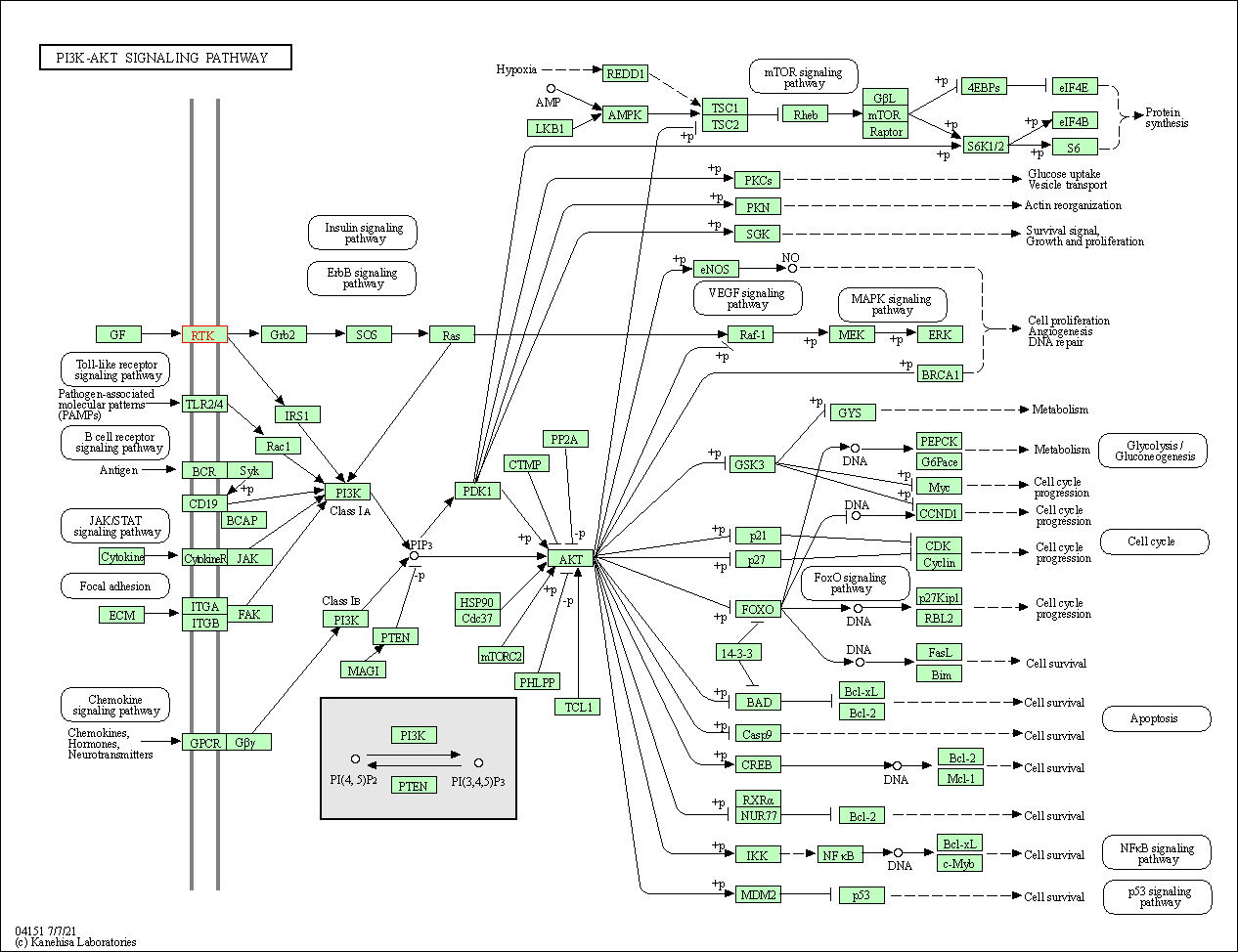
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
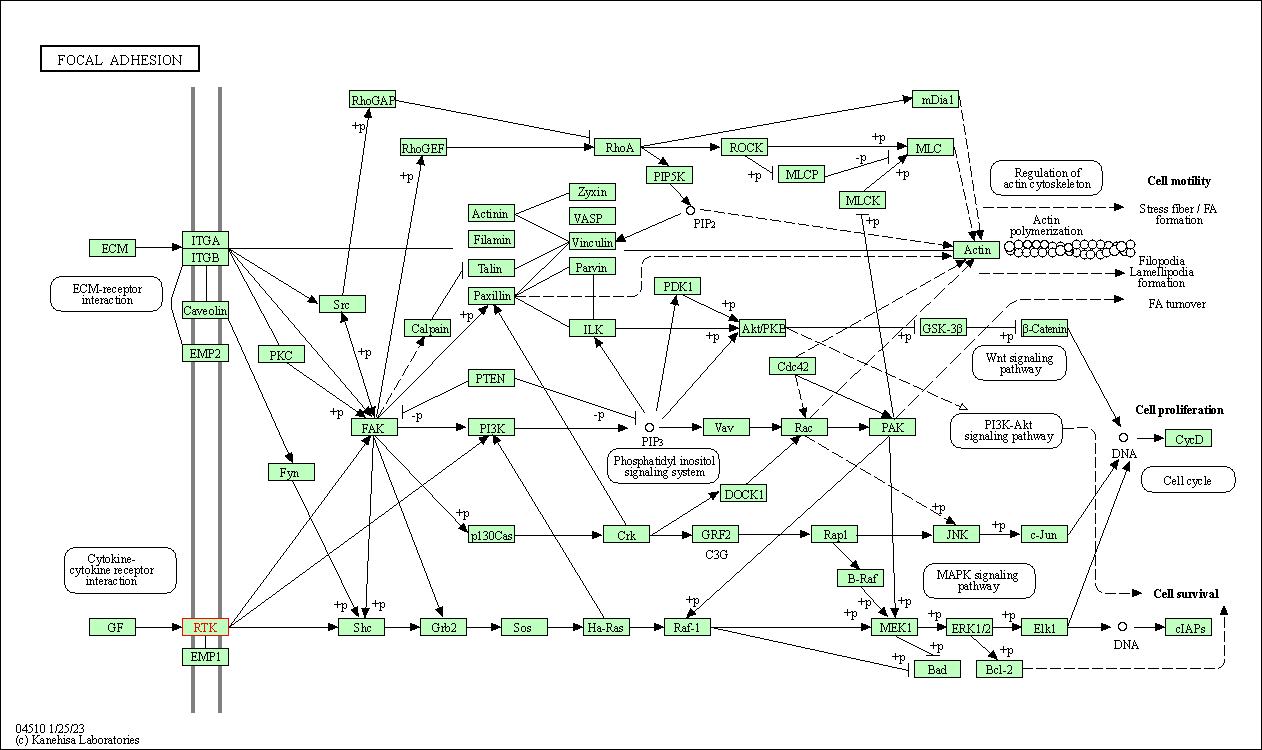
| Focal adhesion | hsa04510 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
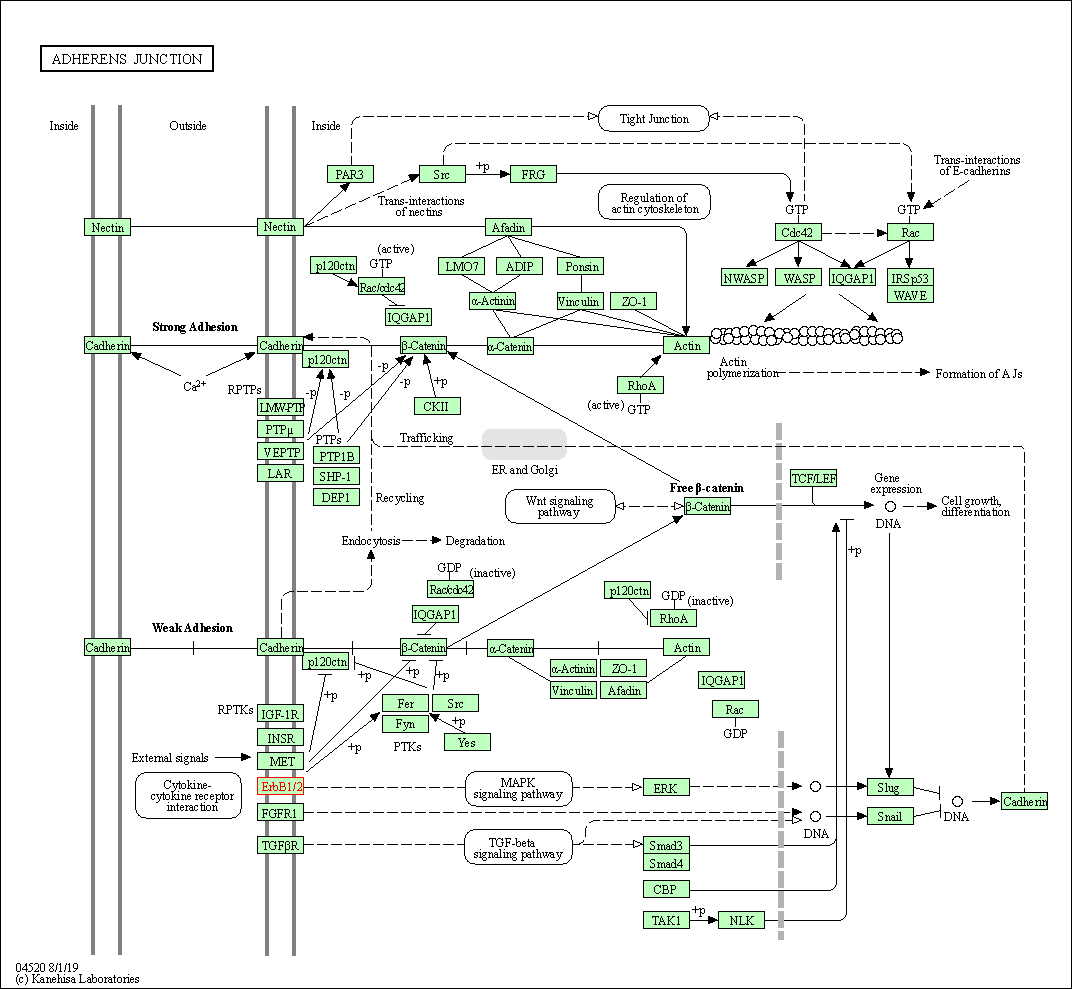
| Adherens junction | hsa04520 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Gap junction | hsa04540 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Regulation of actin cytoskeleton | hsa04810 | Affiliated Target |

|
| Class: Cellular Processes => Cell motility | Pathway Hierarchy | ||
| GnRH signaling pathway | hsa04912 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Estrogen signaling pathway | hsa04915 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Oxytocin signaling pathway | hsa04921 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Relaxin signaling pathway | hsa04926 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Parathyroid hormone synthesis, secretion and action | hsa04928 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01454596) CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients With Malignant Gliomas Expressing EGFRvIII. U.S. National Institutes of Health. | |||||
| REF 2 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 5 | ClinicalTrials.gov (NCT02844062) Pilot Study of Autologous Anti-EGFRvIII CAR T Cells in Recurrent Glioblastoma Multiforme | |||||
| REF 6 | ClinicalTrials.gov (NCT03267173) Evaluate the Safety and Efficacy of CAR-T in the Treatment of Pancreatic Cancer. | |||||
| REF 7 | ClinicalTrials.gov (NCT03170141) Immunogene-modified T (IgT) Cells Against Glioblastoma Multiforme | |||||
| REF 8 | ClinicalTrials.gov (NCT03726515) CART-EGFRvIII + Pembrolizumab in GBM | |||||
| REF 9 | ClinicalTrials.gov (NCT02303678) D2C7 for Adult Patients With Recurrent Malignant Glioma. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02664363) EGFRvIII CAR T Cells for Newly-Diagnosed WHO Grade IV Malignant Glioma | |||||
| REF 11 | ClinicalTrials.gov (NCT03283631) Intracerebral EGFR-vIII CAR-T Cells for Recurrent GBM | |||||
| REF 12 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 13 | EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014 Feb 15;20(4):972-84. | |||||
| REF 14 | Clinical pipeline report, company report or official report of Amgen. | |||||
| REF 15 | ClinicalTrials.gov (NCT02209376) Autologous T Cells Redirected to EGFRVIII-With a Chimeric Antigen Receptor in Patients With EGFRVIII+ Glioblastoma | |||||
| REF 16 | Improved efficacy against malignant brain tumors with EGFRwt/EGFRvIII targeting immunotoxin and checkpoint inhibitor combinations. J Immunother Cancer. 2019 May 29;7(1):142. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

