Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T66693
(Former ID: TTDR00934)
|
|||||
| Target Name |
B-cell maturation protein (TNFRSF17)
|
|||||
| Synonyms |
Tumor necrosis factor receptor superfamily member 17; CD269; BCMA; BCM
Click to Show/Hide
|
|||||
| Gene Name |
TNFRSF17
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Multiple myeloma [ICD-11: 2A83] | |||||
| Function |
Promotes B-cell survival and plays a role in the regulation of humoral immunity. Activates NF-kappa-B and JNK. Receptor for TNFSF13B/BLyS/BAFF and TNFSF13/APRIL.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MLQMAGQCSQNEYFDSLLHACIPCQLRCSSNTPPLTCQRYCNASVTNSVKGTNAILWTCL
GLSLIISLAVFVLMFLLRKINSEPLKDEFKNTGSGLLGMANIDLEKSRTGDEIILPRGLE YTVEECTCEDCIKSKPKVDSDHCFPLPAMEEGATILVTTKTNDYCKSLPAALSATEIEKS ISAR Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Belantamab mafodotin | Drug Info | Approved | Multiple myeloma | [2] | |
| 2 | Elranatamab | Drug Info | Approved | Multiple myeloma | [3] | |
| 3 | Idecabtagene vicleucel | Drug Info | Approved | Multiple myeloma | [4] | |
| 4 | Teclistamab | Drug Info | Approved | Multiple myeloma | [5] | |
| Clinical Trial Drug(s) | [+] 63 Clinical Trial Drugs | + | ||||
| 1 | Ciltacabtagene autoleucel | Drug Info | Phase 3 | Multiple myeloma | [6] | |
| 2 | Bb2121 | Drug Info | Phase 2 | Multiple myeloma | [7], [8] | |
| 3 | Descartes-08 | Drug Info | Phase 2 | Multiple myeloma | [9] | |
| 4 | Descartes-11 | Drug Info | Phase 2 | Multiple myeloma | [10] | |
| 5 | LCAR-B38M CAR-T Cell | Drug Info | Phase 2 | Multiple myeloma | [1], [11] | |
| 6 | Anti-BCMA CAR-T cells | Drug Info | Phase 1/2 | Multiple myeloma | [12] | |
| 7 | Anti-BCMA-CAR-transduced T cells | Drug Info | Phase 1/2 | leukaemia | [13] | |
| 8 | AUTO2 | Drug Info | Phase 1/2 | Multiple myeloma | [14] | |
| 9 | BCMA CAR T cells | Drug Info | Phase 1/2 | Multiple myeloma | [15] | |
| 10 | BCMA CAR-T | Drug Info | Phase 1/2 | Multiple myeloma | [16] | |
| 11 | CAR-T cells targeting BCMA | Drug Info | Phase 1/2 | leukaemia | [17] | |
| 12 | CART-19/BCMA | Drug Info | Phase 1/2 | Multiple myeloma | [18] | |
| 13 | CART-BCMA cells | Drug Info | Phase 1/2 | Multiple myeloma | [19] | |
| 14 | CD38 and BCMA CAR-T Cells | Drug Info | Phase 1/2 | Multiple myeloma | [20] | |
| 15 | JCARH125 | Drug Info | Phase 1/2 | Multiple myeloma | [21], [22] | |
| 16 | JNJ-68284528 | Drug Info | Phase 1/2 | Multiple myeloma | [23] | |
| 17 | PBCAR269A | Drug Info | Phase 1/2 | Multiple myeloma | [24] | |
| 18 | REGN5458 | Drug Info | Phase 1/2 | Multiple myeloma | [25] | |
| 19 | REGN5459 | Drug Info | Phase 1/2 | Multiple myeloma | [26] | |
| 20 | ALLO-715 | Drug Info | Phase 1 | Multiple myeloma | [27] | |
| 21 | AMG 420 | Drug Info | Phase 1 | Multiple myeloma | [7], [8] | |
| 22 | AMG 701 | Drug Info | Phase 1 | Multiple myeloma | [7] | |
| 23 | Anti-BCMA CAR T cells | Drug Info | Phase 1 | Multiple myeloma | [28] | |
| 24 | Anti-BCMA CAR T cells | Drug Info | Phase 1 | Multiple myeloma | [29], [30] | |
| 25 | Anti-BCMA CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [31] | |
| 26 | Anti-BCMA CAR-T cells | Drug Info | Phase 1 | leukaemia | [32] | |
| 27 | Anti-BCMA CART Cells | Drug Info | Phase 1 | Multiple myeloma | [33] | |
| 28 | Anti-CD19/BCMA CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [34] | |
| 29 | Autologous Anti-BCMA-CAR-expressing CD4+/CD8+ T-lymphocytes FCARH143 | Drug Info | Phase 1 | Plasma cell myeloma | [35] | |
| 30 | Bb21217 | Drug Info | Phase 1 | Multiple myeloma | [36] | |
| 31 | BCMA CAR-T Cells | Drug Info | Phase 1 | Multiple myeloma | [37] | |
| 32 | BCMA CART | Drug Info | Phase 1 | Multiple myeloma | [38] | |
| 33 | BCMA CART and huCART19 | Drug Info | Phase 1 | Multiple myeloma | [38] | |
| 34 | BCMA nanobody CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [39] | |
| 35 | BCMA-CD19 cCAR | Drug Info | Phase 1 | Multiple myeloma | [40] | |
| 36 | BCMA-CS1 cCAR | Drug Info | Phase 1 | Multiple myeloma | [41] | |
| 37 | BCMA-specific CAR-expressing T Lymphocytes | Drug Info | Phase 1 | Plasma cell myeloma | [42] | |
| 38 | C-CAR088 | Drug Info | Phase 1 | Multiple myeloma | [43] | |
| 39 | CAR-T cells targeting BCMA | Drug Info | Phase 1 | Multiple myeloma | [44] | |
| 40 | CART-BCMA | Drug Info | Phase 1 | Multiple myeloma | [45] | |
| 41 | CART-ddBCMA | Drug Info | Phase 1 | Multiple myeloma | [46] | |
| 42 | CC-93269 | Drug Info | Phase 1 | Multiple myeloma | [7] | |
| 43 | CC-98633 | Drug Info | Phase 1 | Multiple myeloma | [47] | |
| 44 | CC-99712 | Drug Info | Phase 1 | Multiple myeloma | [48] | |
| 45 | CT053 | Drug Info | Phase 1 | Multiple myeloma | [49] | |
| 46 | CTX120 | Drug Info | Phase 1 | Multiple myeloma | [50] | |
| 47 | HPN217 | Drug Info | Phase 1 | Multiple myeloma | [51] | |
| 48 | IM21 CART | Drug Info | Phase 1 | Multiple myeloma | [52] | |
| 49 | KITE-585 | Drug Info | Phase 1 | Multiple myeloma | [7] | |
| 50 | LCAR-B4822M CAR-T Cell | Drug Info | Phase 1 | Multiple myeloma | [53] | |
| 51 | MEDI2228 | Drug Info | Phase 1 | Multiple myeloma | [54] | |
| 52 | P-BCMA-101 CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [55] | |
| 53 | P-PSMA-101 | Drug Info | Phase 1 | Prostate cancer | [56] | |
| 54 | PHE885 | Drug Info | Phase 1 | Multiple myeloma | [57] | |
| 55 | RG6296 | Drug Info | Phase 1 | Multiple myeloma | [58] | |
| 56 | RG6538 | Drug Info | Phase 1 | Multiple myeloma | [59] | |
| 57 | SEA-BCMA | Drug Info | Phase 1 | Multiple myeloma | [60] | |
| 58 | TNB-383B | Drug Info | Phase 1 | Multiple myeloma | [61] | |
| 59 | WVT078 | Drug Info | Phase 1 | Multiple myeloma | [62] | |
| 60 | BCMA-CART | Drug Info | Clinical trial | Multiple myeloma | [63] | |
| 61 | BCMA-UCART | Drug Info | Clinical trial | Multiple myeloma | [64] | |
| 62 | CAR-BCMA T cell | Drug Info | Clinical trial | Multiple myeloma | [65] | |
| 63 | CAR-T cells targeting BCMA | Drug Info | Clinical trial | Multiple myeloma | [66] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | P-BCMA-ALL01 | Drug Info | Preclinical | Multiple myeloma | [67] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| CAR-T-Cell-Therapy | [+] 33 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Idecabtagene vicleucel | Drug Info | [4] | |||
| 2 | Descartes-08 | Drug Info | [68] | |||
| 3 | LCAR-B38M CAR-T Cell | Drug Info | [1], [11] | |||
| 4 | Anti-BCMA CAR-T cells | Drug Info | [12] | |||
| 5 | Anti-BCMA-CAR-transduced T cells | Drug Info | [13] | |||
| 6 | BCMA CAR T cells | Drug Info | [15] | |||
| 7 | BCMA CAR-T | Drug Info | [16] | |||
| 8 | CAR-T cells targeting BCMA | Drug Info | [17] | |||
| 9 | CART-BCMA cells | Drug Info | [19] | |||
| 10 | JCARH125 | Drug Info | [21], [22] | |||
| 11 | JNJ-68284528 | Drug Info | [23] | |||
| 12 | Anti-BCMA CAR T cells | Drug Info | [28] | |||
| 13 | Anti-BCMA CAR T cells | Drug Info | [29], [30] | |||
| 14 | Anti-BCMA CAR-T cells | Drug Info | [31] | |||
| 15 | Anti-BCMA CAR-T cells | Drug Info | [32] | |||
| 16 | Anti-BCMA CART Cells | Drug Info | [33] | |||
| 17 | Autologous Anti-BCMA-CAR-expressing CD4+/CD8+ T-lymphocytes FCARH143 | Drug Info | [35] | |||
| 18 | Bb21217 | Drug Info | [36] | |||
| 19 | BCMA CAR-T Cells | Drug Info | [37] | |||
| 20 | BCMA CART | Drug Info | [38] | |||
| 21 | BCMA nanobody CAR-T cells | Drug Info | [39] | |||
| 22 | BCMA-specific CAR-expressing T Lymphocytes | Drug Info | [42] | |||
| 23 | C-CAR088 | Drug Info | [43] | |||
| 24 | CAR-T cells targeting BCMA | Drug Info | [44] | |||
| 25 | CART-BCMA | Drug Info | [45] | |||
| 26 | IM21 CART | Drug Info | [52] | |||
| 27 | KITE-585 | Drug Info | [80] | |||
| 28 | LCAR-B4822M CAR-T Cell | Drug Info | [53] | |||
| 29 | P-BCMA-101 CAR-T cells | Drug Info | [55] | |||
| 30 | BCMA-CART | Drug Info | [63] | |||
| 31 | BCMA-UCART | Drug Info | [64] | |||
| 32 | CAR-BCMA T cell | Drug Info | [65] | |||
| 33 | CAR-T cells targeting BCMA | Drug Info | [66] | |||
| Immunomodulator | [+] 1 Immunomodulator drugs | + | ||||
| 1 | Bb2121 | Drug Info | [8] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 5 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | AUTO2 | Drug Info | [14] | |||
| 2 | CART-19/BCMA | Drug Info | [18] | |||
| 3 | CD38 and BCMA CAR-T Cells | Drug Info | [20] | |||
| 4 | Anti-CD19/BCMA CAR-T cells | Drug Info | [34] | |||
| 5 | BCMA CART and huCART19 | Drug Info | [38] | |||
| Inhibitor | [+] 6 Inhibitor drugs | + | ||||
| 1 | REGN5458 | Drug Info | [71] | |||
| 2 | REGN5459 | Drug Info | [72] | |||
| 3 | AMG 420 | Drug Info | [74] | |||
| 4 | AMG 701 | Drug Info | [75] | |||
| 5 | MEDI2228 | Drug Info | [81] | |||
| 6 | RG6296 | Drug Info | [83] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
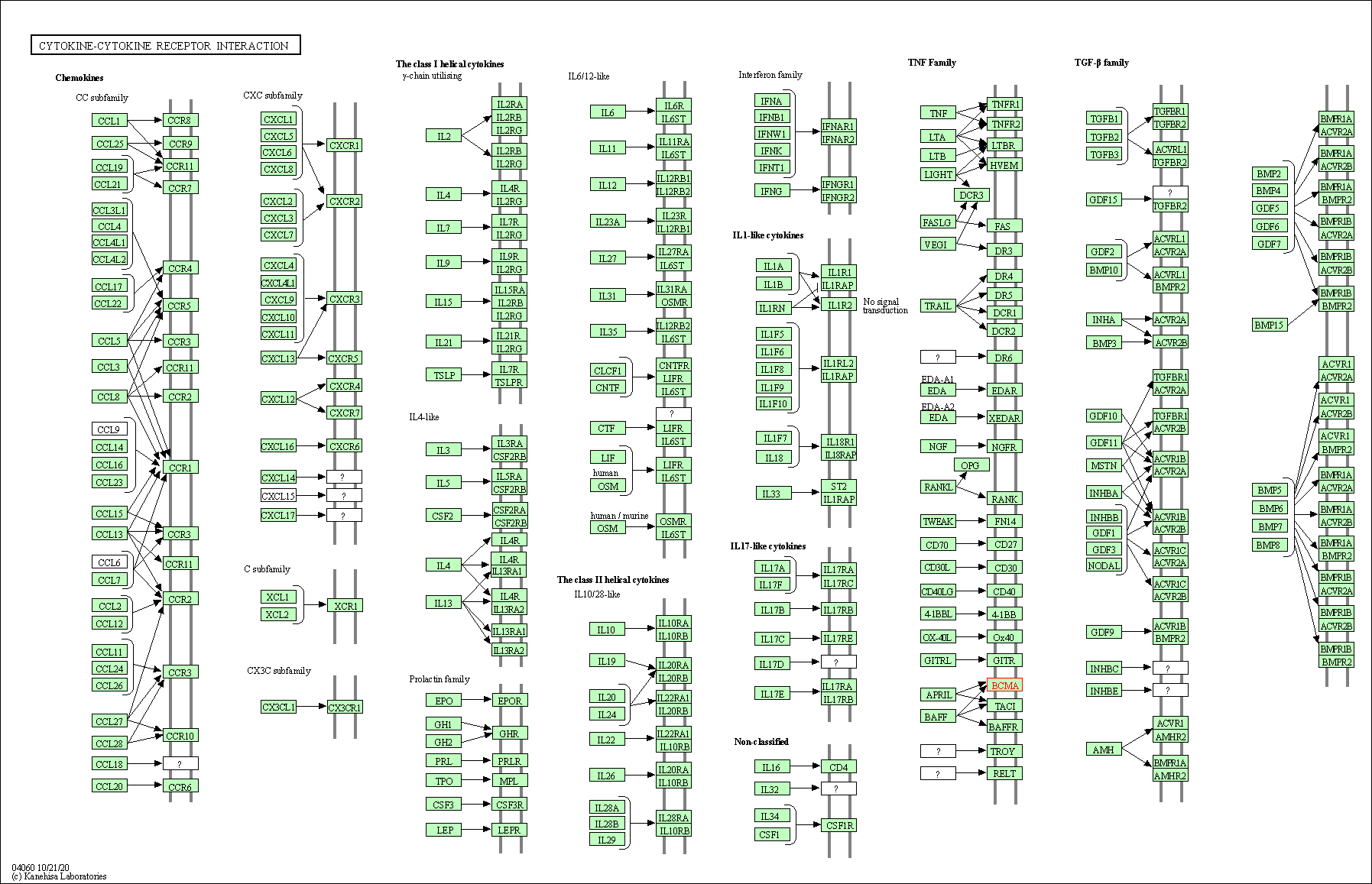
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |
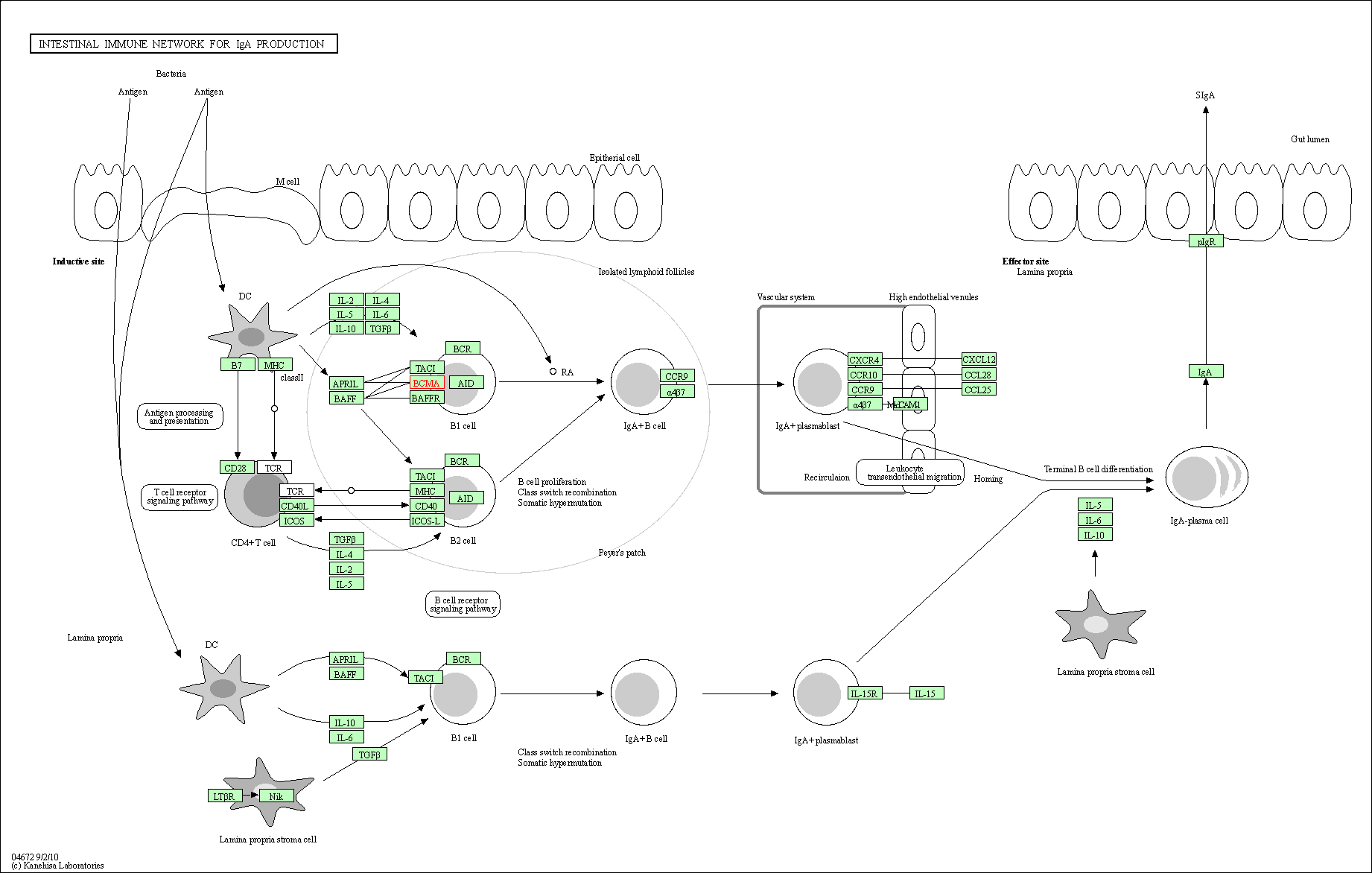
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 5 | Degree centrality | 5.37E-04 | Betweenness centrality | 1.75E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.89E-01 | Radiality | 1.32E+01 | Clustering coefficient | 6.00E-01 |
| Neighborhood connectivity | 1.20E+01 | Topological coefficient | 2.61E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03758417) A Study of LCAR-B38M CAR-T Cells, a Chimeric Antigen Receptor T-cell (CAR-T) Therapy Directed Against B-cell Maturation Antigen (BCMA) in Chinese Participants With Relapsed or Refractory Multiple Myeloma | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 761345 | |||||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. | |||||
| REF 5 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761291. | |||||
| REF 6 | ClinicalTrials.gov (NCT04181827) A Study Comparing JNJ-68284528, a CAR-T Therapy Directed Against B-cell Maturation Antigen (BCMA), Versus Pomalidomide, Bortezomib and Dexamethasone (PVd) or Daratumumab, Pomalidomide and Dexamethasone (DPd) in Participants With Relapsed and Lenalidomide-Refractory Multiple Myeloma (CARTITUDE-4). U.S. National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 9 | ClinicalTrials.gov (NCT04816526) Descartes-08 Consolidation Treatment in Patients With High-Risk Multiple Myeloma Who Have Residual Disease After Induction Therapy. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT04436029) Descartes-11 Consolidation Treatment in Patients With High-Risk Multiple Myeloma Who Have Residual Disease After Induction Therapy. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT03090659) LCAR-B38M-02 Cells in Treating Relapsed/Refractory (R/R) Multiple Myeloma | |||||
| REF 12 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 13 | ClinicalTrials.gov (NCT02954445) A Clinical Research of BCMA-Targeted CAR-T in B Cell Malignancies | |||||
| REF 14 | ClinicalTrials.gov (NCT03287804) APRIL CAR T Cells (AUTO2) Targeting BCMA and TACI for the Treatment of Multiple Myeloma | |||||
| REF 15 | ClinicalTrials.gov (NCT03271632) Multi-CAR T Cell Therapy in the Treatment of Multiple Myeloma | |||||
| REF 16 | ClinicalTrials.gov (NCT03322735) Study of BCMA CAR-T in Multiple Myeloma | |||||
| REF 17 | ClinicalTrials.gov (NCT03312205) CAR-T Cells for Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | |||||
| REF 18 | ClinicalTrials.gov (NCT03455972) Study of T Cells Targeting CD19/BCMA (CART-19/BCMA) for High Risk Multiple Myeloma Followed With Auto-HSCT | |||||
| REF 19 | ClinicalTrials.gov (NCT03196414) Study of CART-138/BCMA Therapy for R/R Multiple Myeloma | |||||
| REF 20 | ClinicalTrials.gov (NCT03767751) A Feasibility and Safety Study of Dual Specificity CD38 and BCMA CAR-T Cell Immunotherapy for Relapsed or Refractory Multiple Myeloma | |||||
| REF 21 | ClinicalTrials.gov (NCT03436771) Long-term Follow-up Study for Patients Previously Treated With a Juno CAR T-Cell Product | |||||
| REF 22 | ClinicalTrials.gov (NCT03430011) Study Evaluating the Safety and Efficacy of JCARH125 in Subjects With Relapsed and/or Refractory Multiple Myeloma | |||||
| REF 23 | ClinicalTrials.gov (NCT03548207) A Study of JNJ-68284528, a Chimeric Antigen Receptor T Cell (CAR-T) Therapy Directed Against B-Cell Maturation Antigen (BCMA) in Participants With Relapsed or Refractory Multiple Myeloma | |||||
| REF 24 | ClinicalTrials.gov (NCT04171843) A Dose-escalation Study to Evaluate the Safety and Clinical Activity of PBCAR269A in Study Participants With Relapsed/Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 25 | ClinicalTrials.gov (NCT03761108) First in Human (FIH) Study of REGN5458 in Patients With Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 26 | ClinicalTrials.gov (NCT04083534) First In Human (FIH) Study of REGN5459 in Adult Patients With Relapsed or Refractory Multiple Myeloma (MM). U.S. National Institutes of Health. | |||||
| REF 27 | ClinicalTrials.gov (NCT04093596) Safety and Efficacy of ALLO-715 BCMA Allogenic CAR T Cells in in Adults With Relapsed or Refractory Multiple Myeloma (UNIVERSAL) (UNIVERSAL). U.S. National Institutes of Health. | |||||
| REF 28 | ClinicalTrials.gov (NCT03559764) Study of BCMA CAR-T in Multiple Myeloma | |||||
| REF 29 | ClinicalTrials.gov (NCT02215967) Study of T Cells Targeting B-Cell Maturation Antigen for Previously Treated Multiple Myeloma | |||||
| REF 30 | ClinicalTrials.gov (NCT03602612) T Cells Expressing a Novel Fully-Human Anti-BCMA CAR for Treating Multiple Myeloma | |||||
| REF 31 | ClinicalTrials.gov (NCT03093168) BCMA Chimeric Antigen Receptor Expressing T Cells in Multiple Myeloma | |||||
| REF 32 | ClinicalTrials.gov (NCT03121625) CAR-T Therapy in Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | |||||
| REF 33 | ClinicalTrials.gov (NCT03767725) Anti-BCMA or/and Anti-CD19 CART Cells Treatment of Relapsed Multiple Myeloma | |||||
| REF 34 | ClinicalTrials.gov (NCT03706547) Anti-CD19/BCMA Bispecific CAR-T Cell Therapy for R/R MM | |||||
| REF 35 | ClinicalTrials.gov (NCT03338972) Immunotherapy With BCMA CAR-T Cells in Treating Patients With BCMA Positive Relapsed or Refractory Multiple Myeloma | |||||
| REF 36 | ClinicalTrials.gov (NCT03274219) Study of bb21217 in Multiple Myeloma | |||||
| REF 37 | ClinicalTrials.gov (NCT03661554) BCMA Nano Antibody CAR-T Cells for Patients With Refractory and Relapsed Multiple Myeloma | |||||
| REF 38 | ClinicalTrials.gov (NCT03549442) Up-front CART-BCMA With or Without huCART19 in High-risk Multiple Myeloma | |||||
| REF 39 | ClinicalTrials.gov (NCT03664661) BCMA-CAR-T in Relapsed/Refractory Multiple Myeloma | |||||
| REF 40 | ClinicalTrials.gov (NCT04162353) BCMA-CD19 cCAR in Multiple Myeloma and Plasmacytoid Lymphoma. U.S. National Institutes of Health. | |||||
| REF 41 | ClinicalTrials.gov (NCT04156269) BCMA-CS1 Compound CAR (cCAR) T Cells for Relapsed/Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 42 | ClinicalTrials.gov (NCT03502577) BCMA-Specific CAR T-Cells Combined With a Gamma Secretase Inhibitor (LY3039478) to Treat Relapsed or Persistent Multiple Myeloma | |||||
| REF 43 | ClinicalTrials.gov (NCT03751293) A Study of BCMA-directed CAR-T Cells Treatment in Subjects With r/r Multiple Myeloma | |||||
| REF 44 | ClinicalTrials.gov (NCT03672253) CAR-T Re-treatment for Refractory/Relapsed Multiple Myeloma | |||||
| REF 45 | ClinicalTrials.gov (NCT02546167) CART-BCMA Cells for Multiple Myeloma | |||||
| REF 46 | Clinical pipeline report, company report or official report of Arcellx. | |||||
| REF 47 | ClinicalTrials.gov (NCT04394650) A Study of CC-98633, BCMA-targeted Chimeric Antigen Receptor (CAR) T Cells, in Subjects With Relapsed and/or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 48 | ClinicalTrials.gov (NCT04036461) A Study of CC-99712, a BCMA Antibody-Drug Conjugate, in Subjects With Relapsed and Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 49 | ClinicalTrials.gov (NCT03915184) Clinical Trial to Evaluate CT053 in Patients With Relapsed and/or Refractory Multiple Myeloma (LUMMICAR STUDY 2). U.S. National Institutes of Health. | |||||
| REF 50 | ClinicalTrials.gov (NCT04244656) A Safety and Efficacy Study Evaluating CTX120 in Subjects With Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 51 | ClinicalTrials.gov (NCT04184050) A Phase 1/2 Open-label, Multicenter, Dose Escalation and Dose Expansion Study of the Safety, Tolerability, and PK of HPN217 in Patients With R/R MM. U.S. National Institutes of Health. | |||||
| REF 52 | ClinicalTrials.gov (NCT03711864) Safety and Efficacy of IM21 Car-t Cells in Patients With Recurrent or Refractory BCMA Positive Multiple Myeloma | |||||
| REF 53 | ClinicalTrials.gov (NCT03674463) LCAR-B4822M-02 Cells in Treating Relapsed/Refractory (R/R) Multiple Myeloma | |||||
| REF 54 | ClinicalTrials.gov (NCT03489525) MEDI2228 in Subjects With Relapsed/Refractory Multiple Myeloma (MEDI2228). U.S. National Institutes of Health. | |||||
| REF 55 | ClinicalTrials.gov (NCT03288493) P-BCMA-101 Tscm CAR-T Cells in the Treatment of Patients With Multiple Myeloma (MM) | |||||
| REF 56 | ClinicalTrials.gov (NCT04249947) P-PSMA-101 CAR-T Cells in the Treatment of Subjects With Metastatic Castration-Resistant Prostate Cancer (mCRPC). U.S. National Institutes of Health. | |||||
| REF 57 | ClinicalTrials.gov (NCT04318327) BCMA-directed CAR-T Cell Therapy in Adult Patients With Relapsed and/or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 58 | ClinicalTrials.gov (NCT04434469) A Study Evaluating The Safety And Pharmacokinetics Of Escalating Doses Of RO7297089 In Patients With Relapsed Or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 59 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 60 | ClinicalTrials.gov (NCT03582033) A Phase 1 Study of SEA-BCMA in Patients With Relapsed or Refractory Multiple Myeloma. U.S.National Institutes of Health. | |||||
| REF 61 | ClinicalTrials.gov (NCT03933735) A Study of TNB-383B in Subjects With Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 62 | ClinicalTrials.gov (NCT04123418) A Study of WVT078 in Patients With Multiple Myeloma (MM). U.S. National Institutes of Health. | |||||
| REF 63 | ClinicalTrials.gov (NCT03492268) Safety and Efficacy Evaluation of BCMA-CART for Treating Multiple Myeloma | |||||
| REF 64 | ClinicalTrials.gov (NCT03752541) Efficacy and Safety Evaluation of BCMA-UCART | |||||
| REF 65 | ClinicalTrials.gov (NCT03302403) Clinical Study of Redirected Autologous T Cells With a Chimeric Antigen Receptor in Patients With Malignant Tumors | |||||
| REF 66 | ClinicalTrials.gov (NCT03473496) CAR-T Cells Therapy in Relapsed/Refractory Multiple Myeloma | |||||
| REF 67 | Clinical pipeline report, company report or official report of Poseida Therapeutics. | |||||
| REF 68 | ClinicalTrials.gov (NCT03448978) Autologous CD8+ T-cells Expressing an Anti-BCMA CAR in Patients With Myeloma | |||||
| REF 69 | Clinical pipeline report, company report or official report of Cartesian Therapeutics. | |||||
| REF 70 | Clinical pipeline report, company report or official report of Precision Biosciences. | |||||
| REF 71 | A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells. Blood Adv. 2021 Mar 9;5(5):1291-1304. | |||||
| REF 72 | Clinical pipeline report, company report or official report of Regeneron Pharmaceuticals. | |||||
| REF 73 | Clinical pipeline report, company report or official report of Allogene Therapeutics. | |||||
| REF 74 | Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol. 2020 Mar 10;38(8):775-783. | |||||
| REF 75 | Clinical pipeline report, company report or official report of Amgen. | |||||
| REF 76 | Clinical pipeline report, company report or official report of iCell Gene Therapeutics. | |||||
| REF 77 | T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget. 2020 Nov 10;11(45):4076-4081. | |||||
| REF 78 | Clinical pipeline report, company report or official report of CRISPR Therapeutics. | |||||
| REF 79 | Clinical pipeline report, company report or official report of AbbVie. | |||||
| REF 80 | ClinicalTrials.gov (NCT03318861) A Study Evaluating the Safety and Efficacy of KITE-585 in Subjects With Relapsed/Refractory Multiple Myeloma | |||||
| REF 81 | A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma. Leukemia. 2020 Aug;34(8):2150-2162. | |||||
| REF 82 | Clinical pipeline report, company report or official report of Poseida Therapeutics. | |||||
| REF 83 | Clinical pipeline report, company report or official report of Genentech. | |||||
| REF 84 | Clinical pipeline report, company report or official report of AbbVie. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

