Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T74002
(Former ID: TTDR00322)
|
|||||
| Target Name |
Granulocyte-macrophage colony-stimulating factor (CSF2)
|
|||||
| Synonyms |
Sargramostim; Molgramostin; GM-CSF; Colony-stimulating factor; CSF2; CSF
Click to Show/Hide
|
|||||
| Gene Name |
CSF2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Melanoma [ICD-11: 2C30] | |||||
| Function |
Cytokine that stimulates the growth and differentiation of hematopoietic precursor cells from various lineages, including granulocytes, macrophages, eosinophils and erythrocytes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Growth factor
|
|||||
| UniProt ID | ||||||
| Sequence |
MWLQSLLLLGTVACSISAPARSPSPSTQPWEHVNAIQEARRLLNLSRDTAAEMNETVEVI
SEMFDLQEPTCLQTRLELYKQGLRGSLTKLKGPLTMMASHYKQHCPPTPETSCATQIITF ESFKENLKDFLLVIPFDCWEPVQE Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A03633 ; BADD_A04013 ; BADD_A06316 | |||||
| HIT2.0 ID | T27U96 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Talimogene Laherparepvec | Drug Info | Approved | Melanoma | [2] | |
| Clinical Trial Drug(s) | [+] 12 Clinical Trial Drugs | + | ||||
| 1 | JX-594 | Drug Info | Phase 3 | Hepatocellular carcinoma | [3], [4], [5] | |
| 2 | Cabiralizumab | Drug Info | Phase 2 | Solid tumour/cancer | [4], [3] | |
| 3 | GSK3196165 | Drug Info | Phase 2 | Rheumatoid arthritis | [6] | |
| 4 | KB-003 | Drug Info | Phase 2 | Severe asthma | [7] | |
| 5 | KB002/003 | Drug Info | Phase 2 | Rheumatoid arthritis | [8] | |
| 6 | Lenzilumab | Drug Info | Phase 2 | Coronavirus Disease 2019 (COVID-19) | [9] | |
| 7 | Mavrilimumab | Drug Info | Phase 2 | Rheumatoid arthritis | [10], [11] | |
| 8 | MT203 | Drug Info | Phase 2 | Plaque psoriasis | [12], [13] | |
| 9 | Autologous melanoma cell vaccine | Drug Info | Phase 1 | Solid tumour/cancer | [14] | |
| 10 | CDNA vaccine | Drug Info | Phase 1 | Prostate cancer | [15] | |
| 11 | CGTG-102 | Drug Info | Phase 1 | Solid tumour/cancer | [16] | |
| 12 | MORAb-022 | Drug Info | Phase 1 | Autoimmune diabetes | [17] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | RPK-739 | Drug Info | Terminated | Solid tumour/cancer | [18] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 9 Modulator drugs | + | ||||
| 1 | Talimogene Laherparepvec | Drug Info | [1] | |||
| 2 | JX-594 | Drug Info | [19] | |||
| 3 | KB002/003 | Drug Info | [21] | |||
| 4 | MT203 | Drug Info | [22] | |||
| 5 | Autologous melanoma cell vaccine | Drug Info | [21] | |||
| 6 | CDNA vaccine | Drug Info | [21] | |||
| 7 | CGTG-102 | Drug Info | [23] | |||
| 8 | RPK-739 | Drug Info | [1] | |||
| 9 | human monoclonal antibodies (GM-CSF) | Drug Info | [25] | |||
| Antagonist | [+] 3 Antagonist drugs | + | ||||
| 1 | Cabiralizumab | Drug Info | [4] | |||
| 2 | Lenzilumab | Drug Info | [4] | |||
| 3 | Mavrilimumab | Drug Info | [6] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
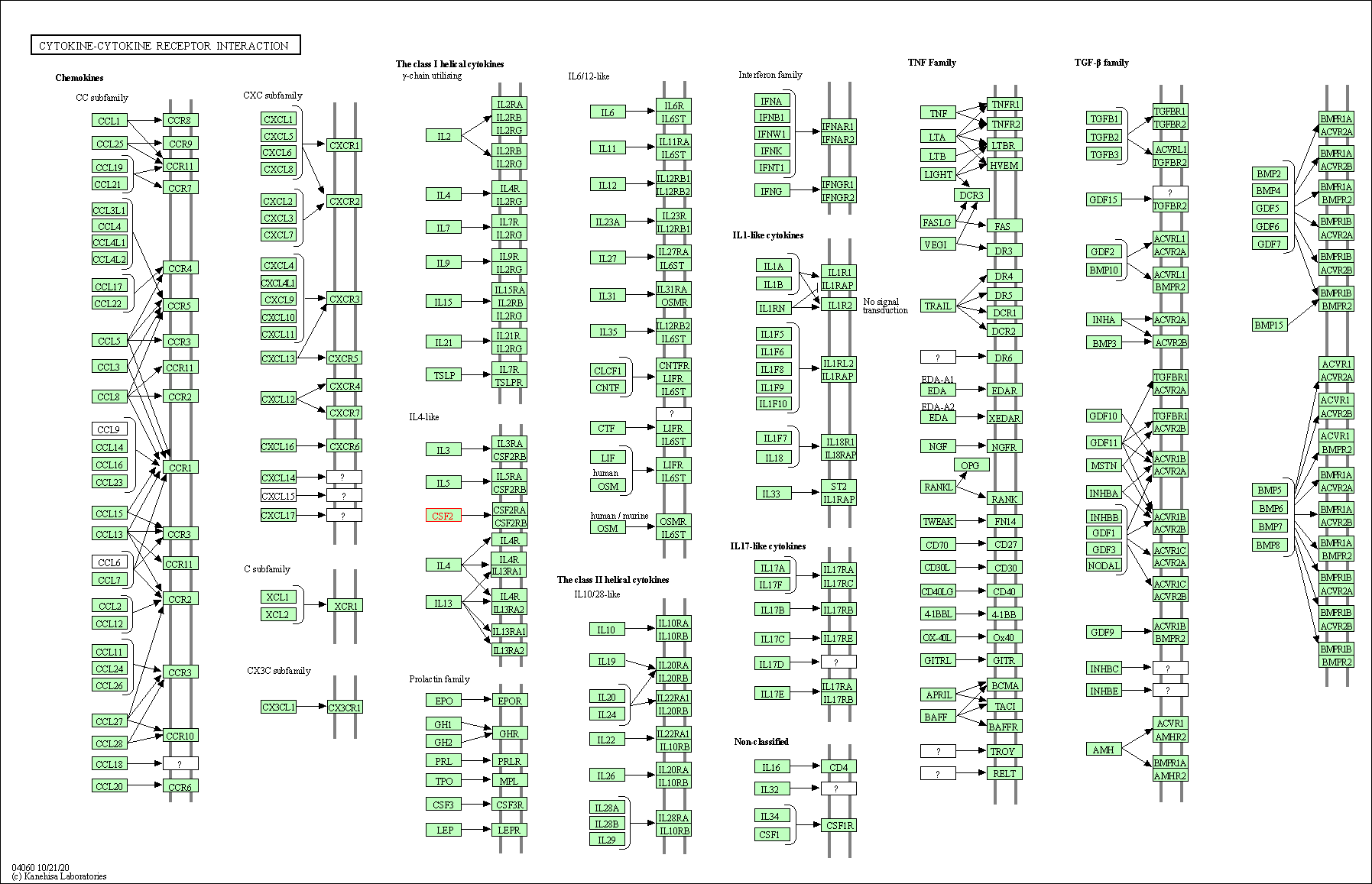
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
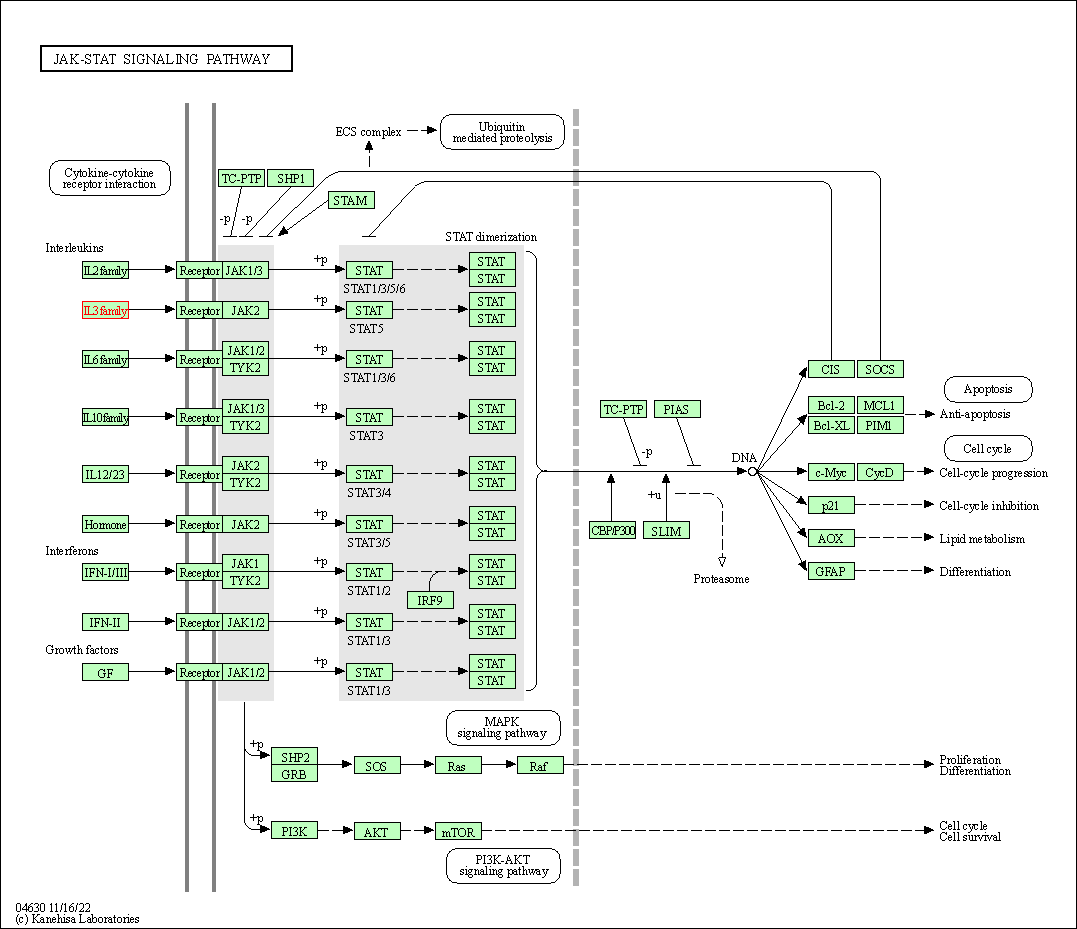
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Natural killer cell mediated cytotoxicity | hsa04650 | Affiliated Target |
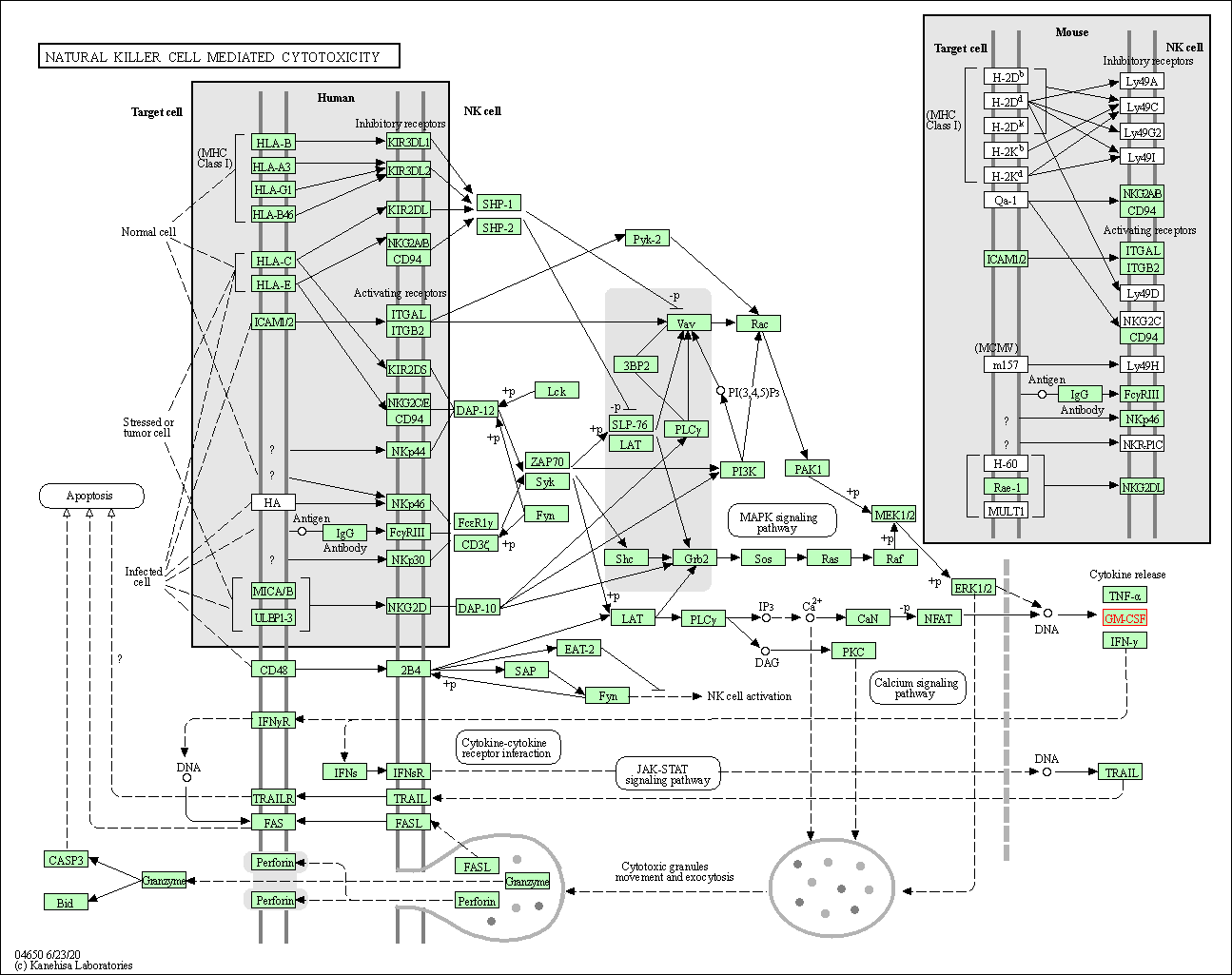
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| IL-17 signaling pathway | hsa04657 | Affiliated Target |
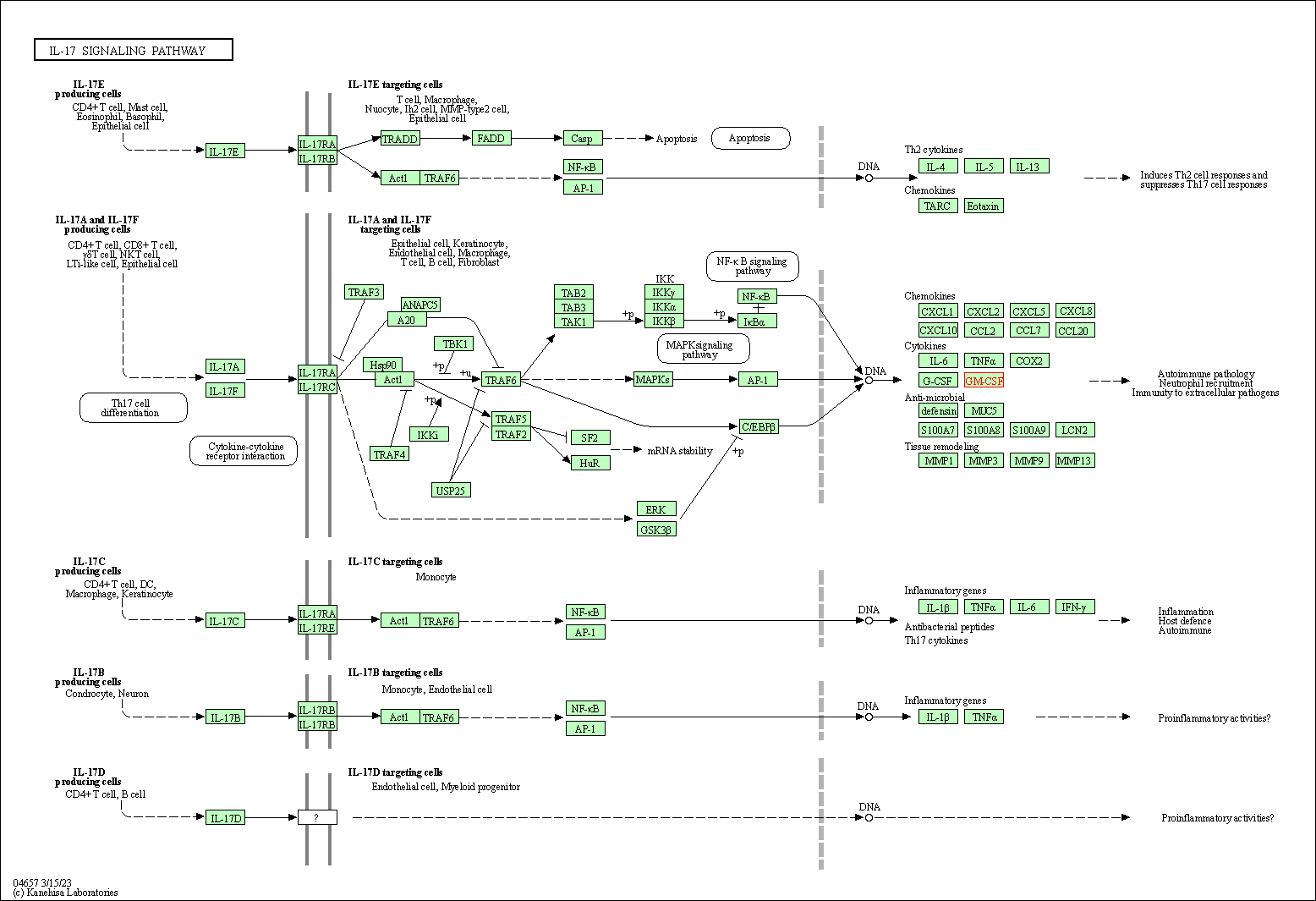
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |
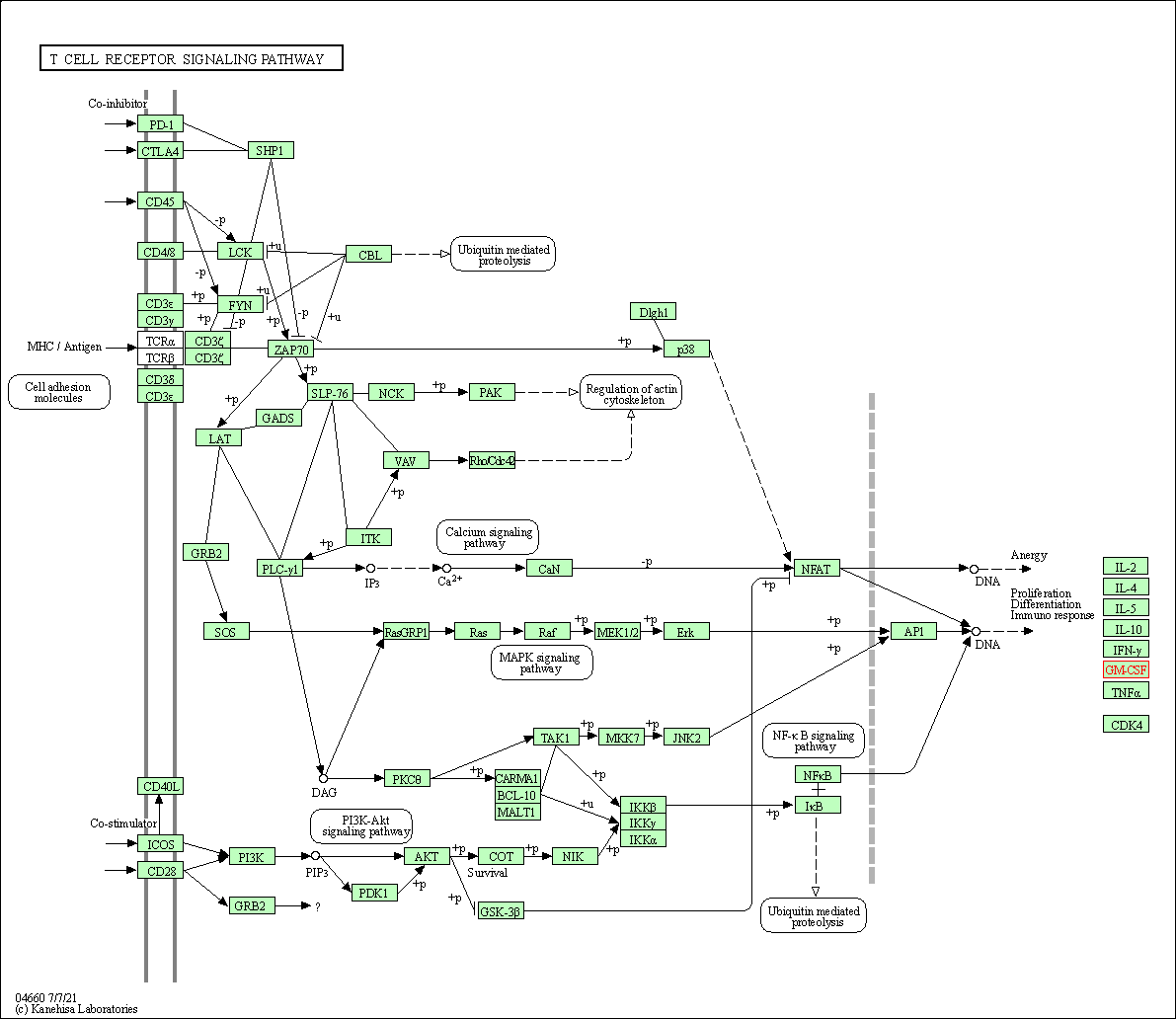
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |
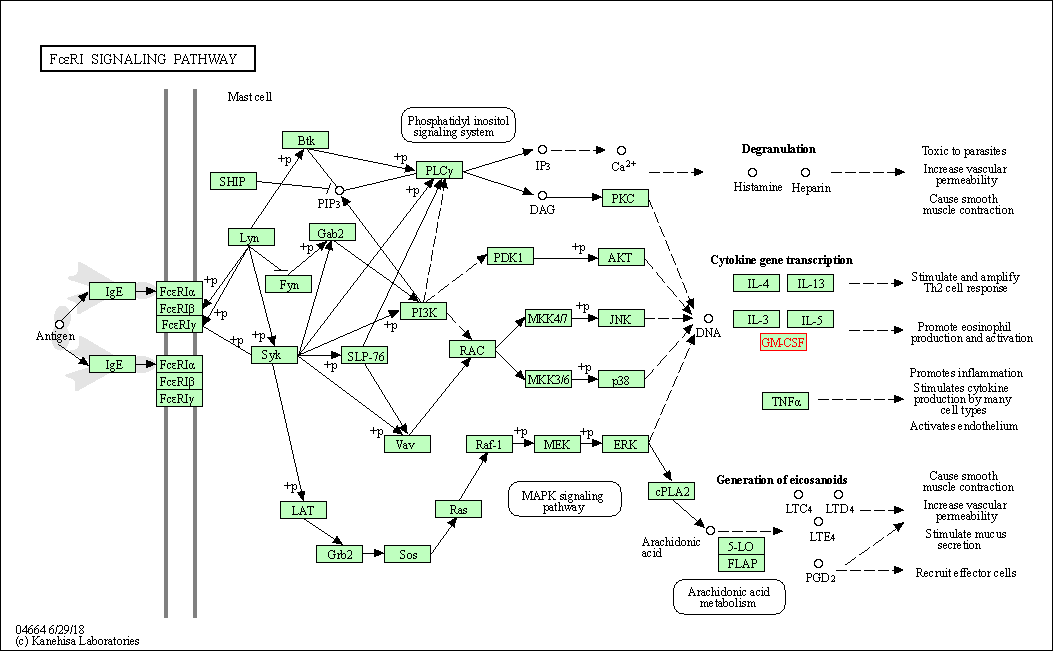
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |
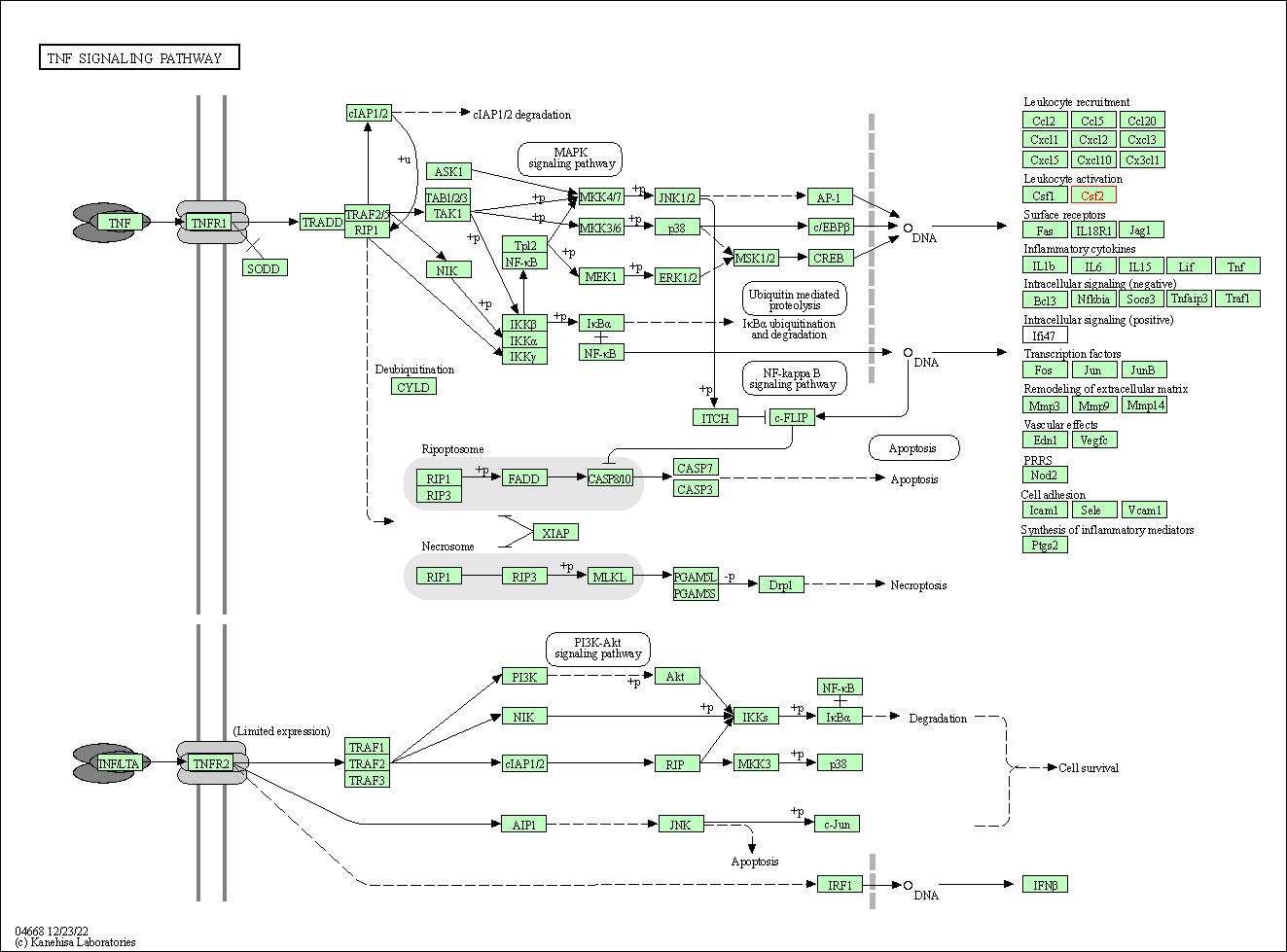
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 31 | Degree centrality | 3.33E-03 | Betweenness centrality | 5.65E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.33E-01 | Radiality | 1.41E+01 | Clustering coefficient | 3.57E-01 |
| Neighborhood connectivity | 3.43E+01 | Topological coefficient | 8.56E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | ClinicalTrials.gov (NCT00995449) Study of KB003 In Biologics-Inadequate Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of KaloBios Pharmaceuticals. | |||||
| REF 9 | ClinicalTrials.gov (NCT04583969) ACTIV-5 / Big Effect Trial (BET-B) for the Treatment of COVID-19. U.S. National Institutes of Health. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7785). | |||||
| REF 11 | Clinical pipeline report, company report or official report of MedImmune (2011). | |||||
| REF 12 | ClinicalTrials.gov (NCT02393378) Namilumab vs Adalimumab in Participants With Moderate to Severe Early Rheumatoid Arthritis Inadequately Responding to Methotrexate. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT02129777) Efficacy and Safety of Namilumab (MT203) for Plaque Psoriasis | |||||
| REF 14 | Clinical pipeline report, company report or official report of Dana-Farber Cancer Institute Inc. | |||||
| REF 15 | Clinical pipeline report, company report or official report of MannKind Corporation. | |||||
| REF 16 | ClinicalTrials.gov (NCT01437280) GOAT; Phase I Open Label Study of CGTG-102, a GM-CSF Encoding Oncolytic Adenovirus, for Advanced Cancers. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT01357759) Safety and Tolerability of MORAb-022 in Healthy and Rheumatoid Arthritis Subjects. U.S. National Institutes of Health. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024125) | |||||
| REF 19 | Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006 Sep;14(3):361-70. | |||||
| REF 20 | US patent application no. 2010,0158,905, Combination therapy of arthritis with tranilast. | |||||
| REF 21 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 22 | GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential. Expert Rev Clin Immunol. 2015 Apr;11(4):457-65. | |||||
| REF 23 | Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans. Int J Cancer. 2014 Aug 1;135(3):720-30. | |||||
| REF 24 | Clinical pipeline report, company report or official report of Morphotek. | |||||
| REF 25 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

