Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T85529
(Former ID: TTDC00208)
|
|||||
| Target Name |
T-cell-specific surface glycoprotein CD28 (CD28)
|
|||||
| Synonyms |
TP44; CD28-S2
Click to Show/Hide
|
|||||
| Gene Name |
CD28
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Transplant rejection [ICD-11: NE84] | |||||
| Function |
Enhances the production of IL4 and IL10 in T-cells in conjunction with TCR/CD3 ligation and CD40L costimulation. Isoform 3 enhances CD40L-mediated activation of NF-kappa-B and kinases MAPK8 and PAK2 in T-cells. Involved in T-cell activation, the induction of cell proliferation and cytokine production and promotion of T-cell survival.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MLRLLLALNLFPSIQVTGNKILVKQSPMLVAYDNAVNLSCKYSYNLFSREFRASLHKGLD
SAVEVCVVYGNYSQQLQVYSKTGFNCDGKLGNESVTFYLQNLYVNQTDIYFCKIEVMYPP PYLDNEKSNGTIIHVKGKHLCPSPLFPGPSKPFWVLVVVGGVLACYSLLVTVAFIIFWVR SKRSRLLHSDYMNMTPRRPGPTRKHYQPYAPPRDFAAYRS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T90QGN | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Belatacept | Drug Info | Approved | Kidney transplant rejection | [2], [3] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | AB-103 | Drug Info | Phase 3 | Necrotizing soft tissue infection | [4] | |
| 2 | Lulizumab pegol | Drug Info | Phase 2 | Systemic lupus erythematosus | [5] | |
| 3 | AU101 | Drug Info | Phase 1/2 | Osteosarcoma | [6], [7] | |
| 4 | REGN5668 | Drug Info | Phase 1/2 | Ovarian cancer | [8] | |
| 5 | REGN5678 | Drug Info | Phase 1/2 | Prostate cancer | [9] | |
| 6 | CD19-CAR and CD28-CAR T Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [10] | |
| 7 | CD28 and CD137 CAR-T Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [11] | |
| 8 | RG6333 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [12] | |
| 9 | RO7443904 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [13] | |
| 10 | SAR442257 | Drug Info | Phase 1 | Malignant neoplasm | [14] | |
| 11 | SAR443216 | Drug Info | Phase 1 | Gastric cancer | [15] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | TGN-1412 | Drug Info | Discontinued in Phase 1 | Autoimmune diabetes | [16] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Inhibitor | [+] 3 Inhibitor drugs | + | ||||
| 1 | Belatacept | Drug Info | [1] | |||
| 2 | REGN5678 | Drug Info | [19] | |||
| 3 | SAR442257 | Drug Info | [21] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | AB-103 | Drug Info | [17], [18] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | Lulizumab pegol | Drug Info | [5] | |||
| Immunomodulator | [+] 1 Immunomodulator drugs | + | ||||
| 1 | AU101 | Drug Info | [6] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 2 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | CD19-CAR and CD28-CAR T Cells | Drug Info | [10] | |||
| 2 | CD28 and CD137 CAR-T Cells | Drug Info | [11] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Cytotoxic T-lymphocyte protein 4 (CTLA-4) | 31.250 (60/192) | 3.93E-16 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
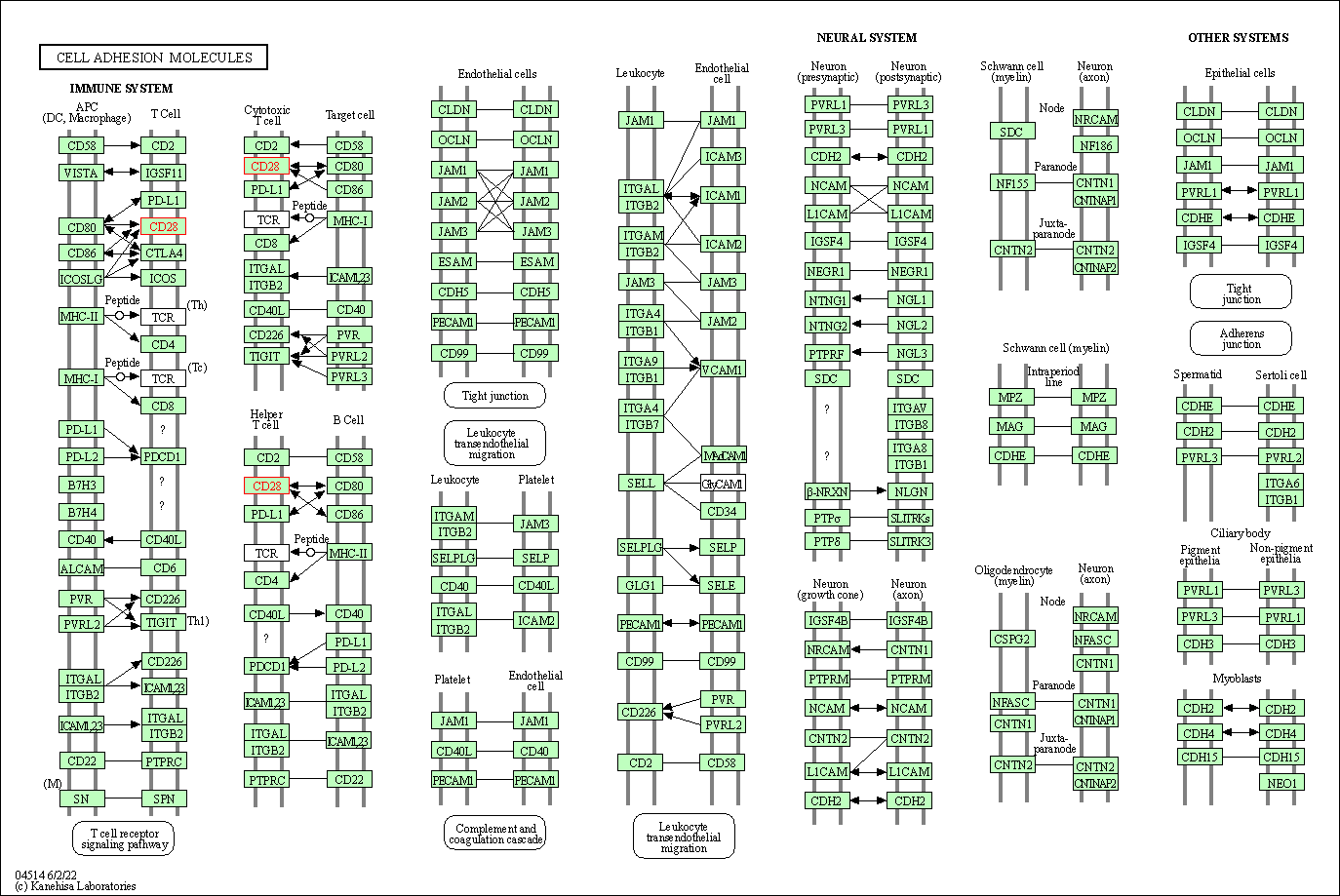
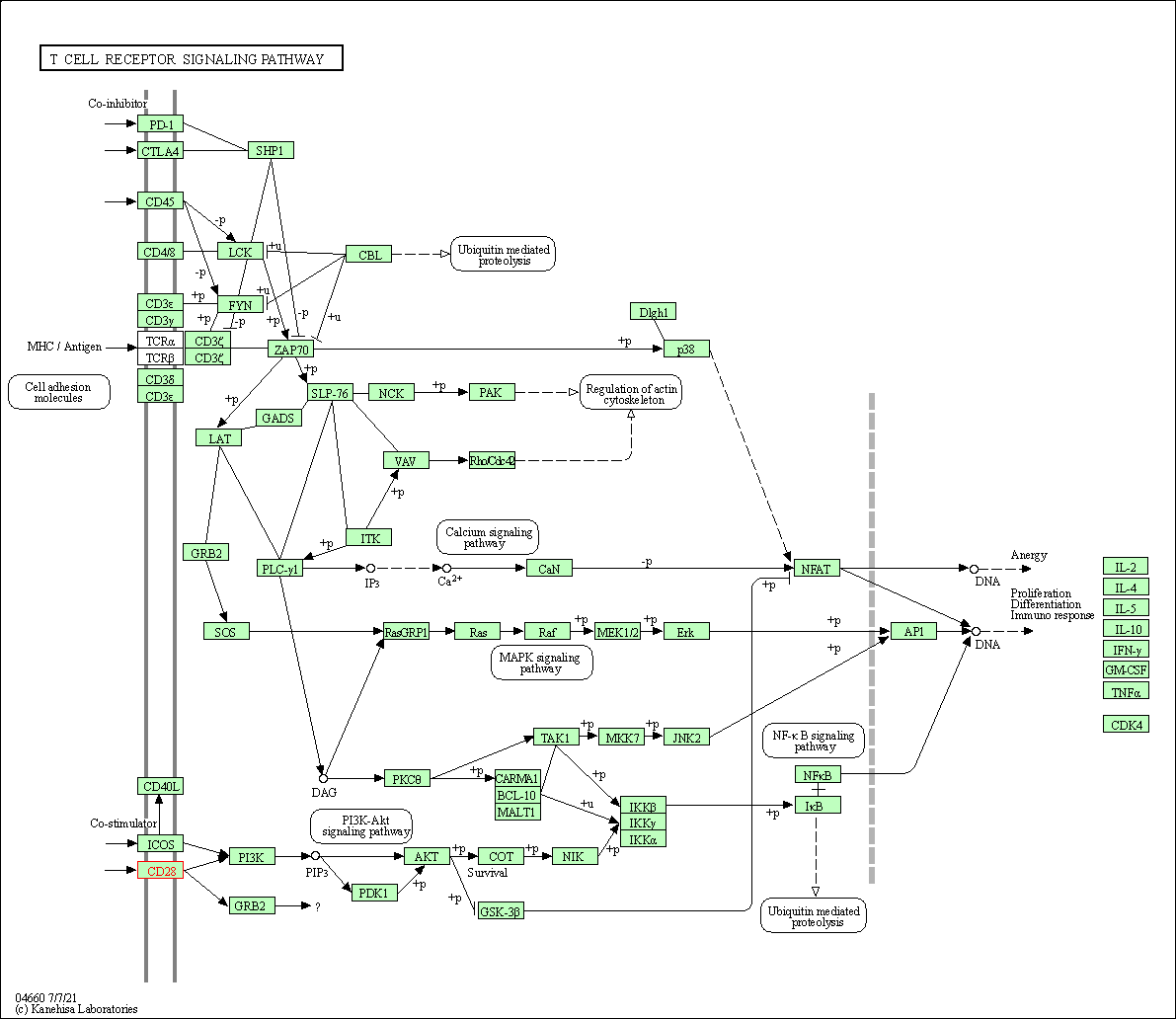
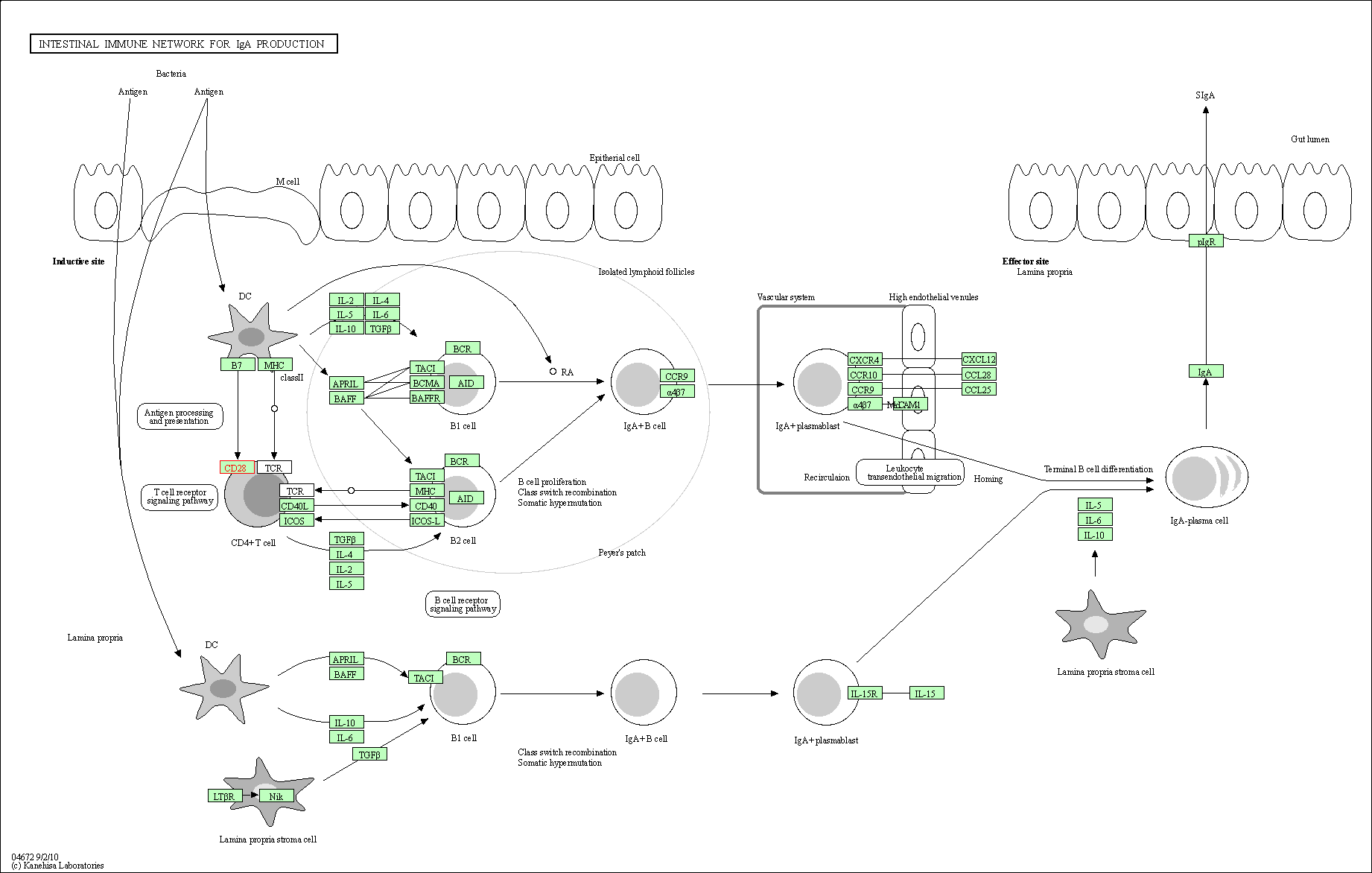
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cell adhesion molecules | hsa04514 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 24 | Degree centrality | 2.58E-03 | Betweenness centrality | 3.03E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.26E-01 | Radiality | 1.40E+01 | Clustering coefficient | 2.93E-01 |
| Neighborhood connectivity | 2.93E+01 | Topological coefficient | 9.17E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | New developments in immunosuppressive therapy for heart transplantation. Expert Opin Emerg Drugs. 2009 Mar;14(1):1-21. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6892). | |||||
| REF 3 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 4 | ClinicalTrials.gov (NCT02469857) Phase III Efficacy and Safety Study of AB103 in the Treatment of Patients With Necrotizing Soft Tissue Infections. | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 8 | ClinicalTrials.gov (NCT04590326) Phase 1/2 Study of REGN5668 (MUC16xCD28, a Costimulatory Bispecific) Administered in Combination With Cemiplimab or REGN4018 (MUC16xCD3). U.S.National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT03972657) Study of REGN5678 (Anti-PSMAxCD28) With Cemiplimab (Anti-PD-1) in Patients With Metastatic Castration-resistant Prostate Cancer. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02146924) Cellular Immunotherapy in Treating Patients With High-Risk Acute Lymphoblastic Leukemia | |||||
| REF 11 | ClinicalTrials.gov (NCT02186860) Chimeric Antigen Receptor (CAR)-Modified T Cell Therapy in Treating Patients With Acute Lymphoblastic Leukemia | |||||
| REF 12 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 13 | ClinicalTrials.gov (NCT05219513) An Open-Label, Phase 1 Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of RO7443904 in Combination With Glofitamab in Participants With Relapsed/Refractory B-Cell Non-Hodgkin's Lymphoma. U.S.National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT04401020) First-in-human Single Agent Study of SAR442257 in RRMM and RR-NHL. U.S. National Institutes of Health. | |||||
| REF 15 | Clinical pipeline report, company report or official report of Sanofi | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020365) | |||||
| REF 17 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 18 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 19 | Clinical pipeline report, company report or official report of Regeneron Pharmaceuticals. | |||||
| REF 20 | Clinical pipeline report, company report or official report of Genentch | |||||
| REF 21 | Clinical pipeline report, company report or official report of Sanofi. | |||||
| REF 22 | TGN1412: From Discovery to Disaster. J Young Pharm. 2010 Jul-Sep; 2(3): 332-336. | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2863). | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

