Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T86552
(Former ID: TTDNC00502)
|
|||||
| Target Name |
Tumor necrosis factor receptor type I (TNF-R1)
|
|||||
| Synonyms |
p60; p55; Tumor necrosis factor-binding protein 1; Tumor necrosis factor receptor superfamily member 1A; Tumor necrosis factor receptor 1; TNFR1; TNFR-I; TNFAR; TNF-RI; TBPI; CD120a
Click to Show/Hide
|
|||||
| Gene Name |
TNFRSF1A
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Brain cancer [ICD-11: 2A00] | |||||
| 2 | Ovarian cancer [ICD-11: 2C73] | |||||
| Function |
The adapter molecule FADD recruits caspase-8 to the activated receptor. The resulting death-inducing signaling complex (DISC) performs caspase-8 proteolytic activation which initiates the subsequent cascade of caspases (aspartate-specific cysteine proteases) mediating apoptosis. Contributes to the induction of non-cytocidal TNF effects including anti-viral state and activation of the acid sphingomyelinase. Receptor for TNFSF2/TNF-alpha and homotrimeric TNFSF1/lymphotoxin-alpha.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MGLSTVPDLLLPLVLLELLVGIYPSGVIGLVPHLGDREKRDSVCPQGKYIHPQNNSICCT
KCHKGTYLYNDCPGPGQDTDCRECESGSFTASENHLRHCLSCSKCRKEMGQVEISSCTVD RDTVCGCRKNQYRHYWSENLFQCFNCSLCLNGTVHLSCQEKQNTVCTCHAGFFLRENECV SCSNCKKSLECTKLCLPQIENVKGTEDSGTTVLLPLVIFFGLCLLSLLFIGLMYRYQRWK SKLYSIVCGKSTPEKEGELEGTTTKPLAPNPSFSPTPGFTPTLGFSPVPSSTFTSSSTYT PGDCPNFAAPRREVAPPYQGADPILATALASDPIPNPLQKWEDSAHKPQSLDTDDPATLY AVVENVPPLRWKEFVRRLGLSDHEIDRLELQNGRCLREAQYSMLATWRRRTPRREATLEL LGRVLRDMDLLGCLEDIEEALCGPAALPPAPSLLR Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A01877 ; BADD_A05438 | |||||
| HIT2.0 ID | T16KVU | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 4 Clinical Trial Drugs | + | ||||
| 1 | VB-111 | Drug Info | Phase 3 | Malignant glioma | [2] | |
| 2 | Drug 2862277 | Drug Info | Phase 2 | Acute lung injury | [3] | |
| 3 | AVX-470 | Drug Info | Phase 1 | Inflammatory bowel disease | [4] | |
| 4 | GSK1995057 | Drug Info | Phase 1 | Adult respiratory distress syndrome | [5] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | Anti-IFN gamma | Drug Info | Terminated | Alopecia | [6] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | VB-111 | Drug Info | [1] | |||
| 2 | ALF-421 | Drug Info | [8] | |||
| 3 | NM-2014 | Drug Info | [7] | |||
| 4 | Recombinant human TNF receptor | Drug Info | [8] | |||
| 5 | TNFcept | Drug Info | [8] | |||
| 6 | TNFR1 NAM | Drug Info | [7] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 5-(3-Morpholin-4-YL-propyl)-2-(3-nitro-phenyl)-4-thioxo-4,5-dihydro-1-thia-3B,5-diaza-cyclopenta[A]pentalen-6-one | Ligand Info | |||||
| Structure Description | PHOTOCHEMICALLY-ENHANCED BINDING OF SMALL MOLECULES TO THE TUMOR NECROSIS FACTOR RECEPTOR-1 | PDB:1FT4 | ||||
| Method | X-ray diffraction | Resolution | 2.90 Å | Mutation | No | [10] |
| PDB Sequence |
MDSVCPQGKY
20 IHPQNNSICC30 TKCHKGTYLY40 NDCPGPGQDT50 DCRECESGSF60 TASENHLRHC 70 LSCSKCRKEM80 GQVEISSCTV90 DRDTVCGCRK100 NQYRHYWSEN110 LFQCFNCSLC 120 LNGTVHLSCQ130 EKQNTVCTCH140 AGFFLRENEC150
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Protein FAM186A (FAM186A) | 29.000 (29/100) | 5.00E-03 | |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
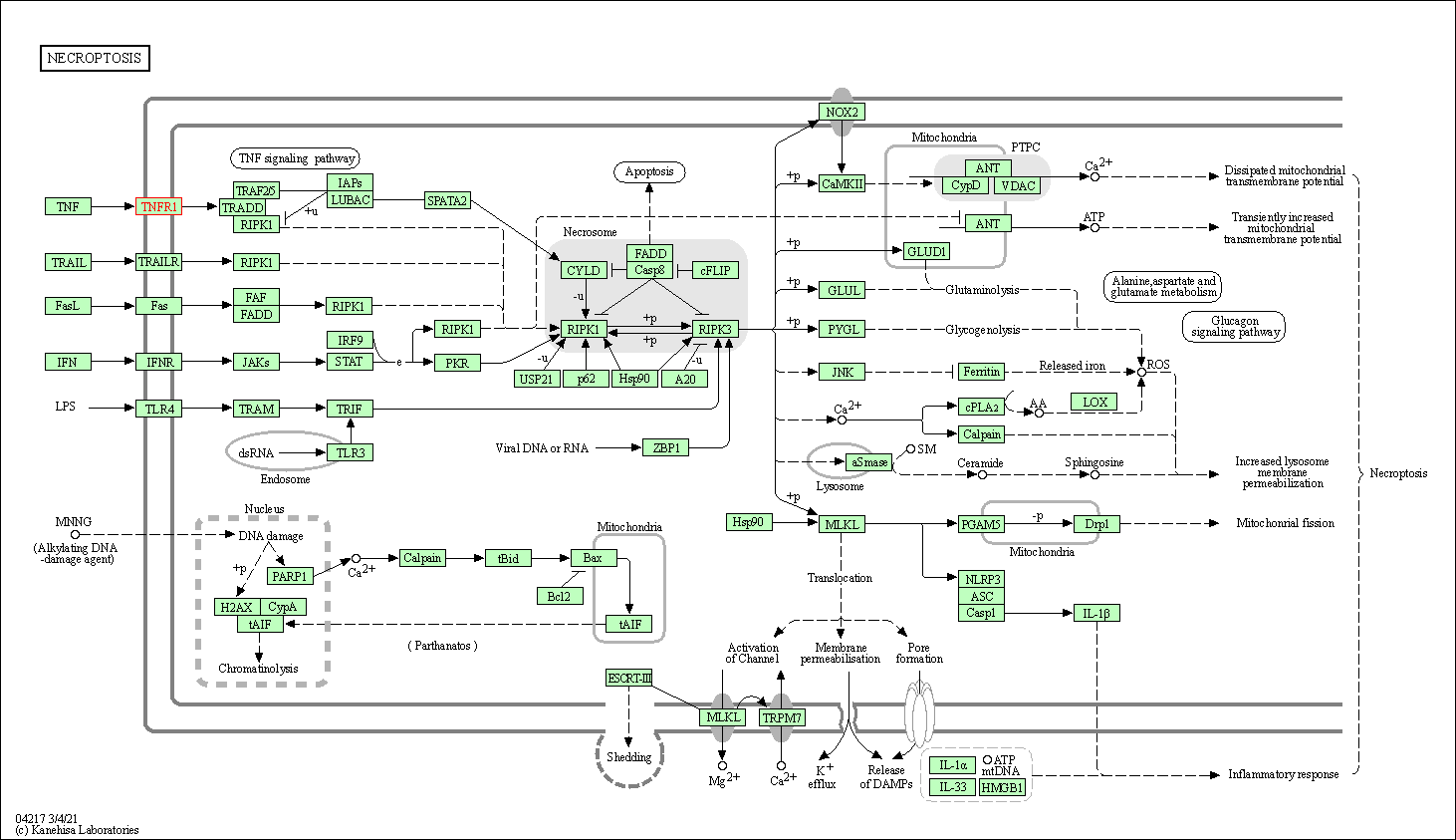
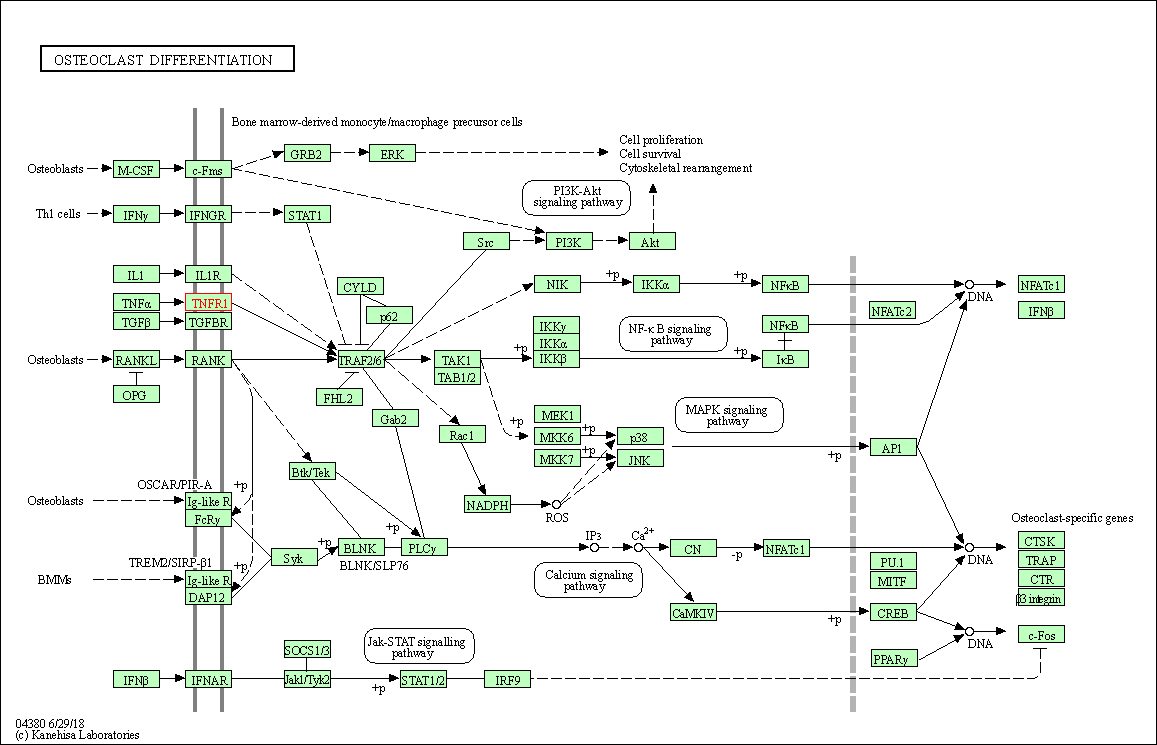
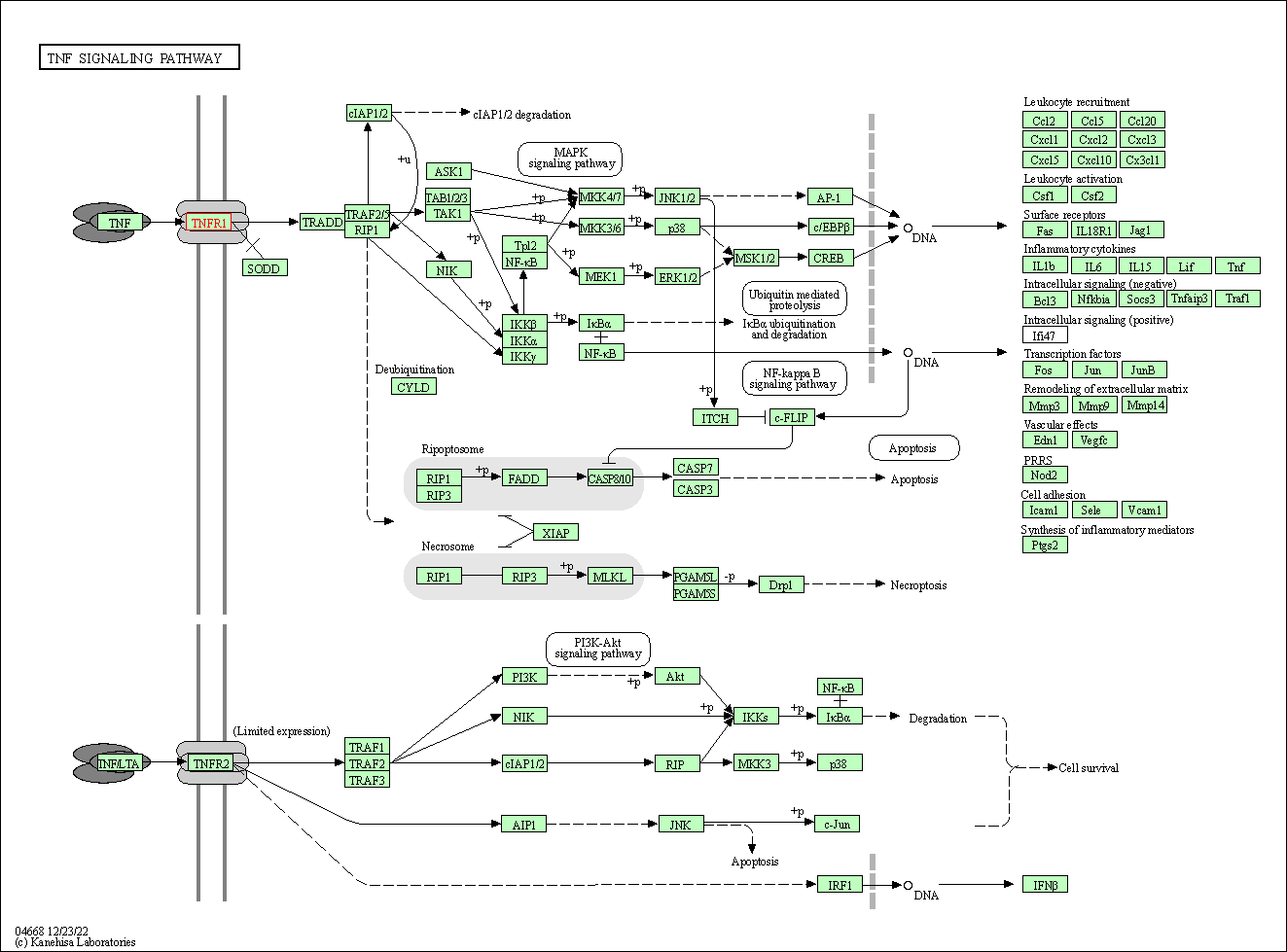
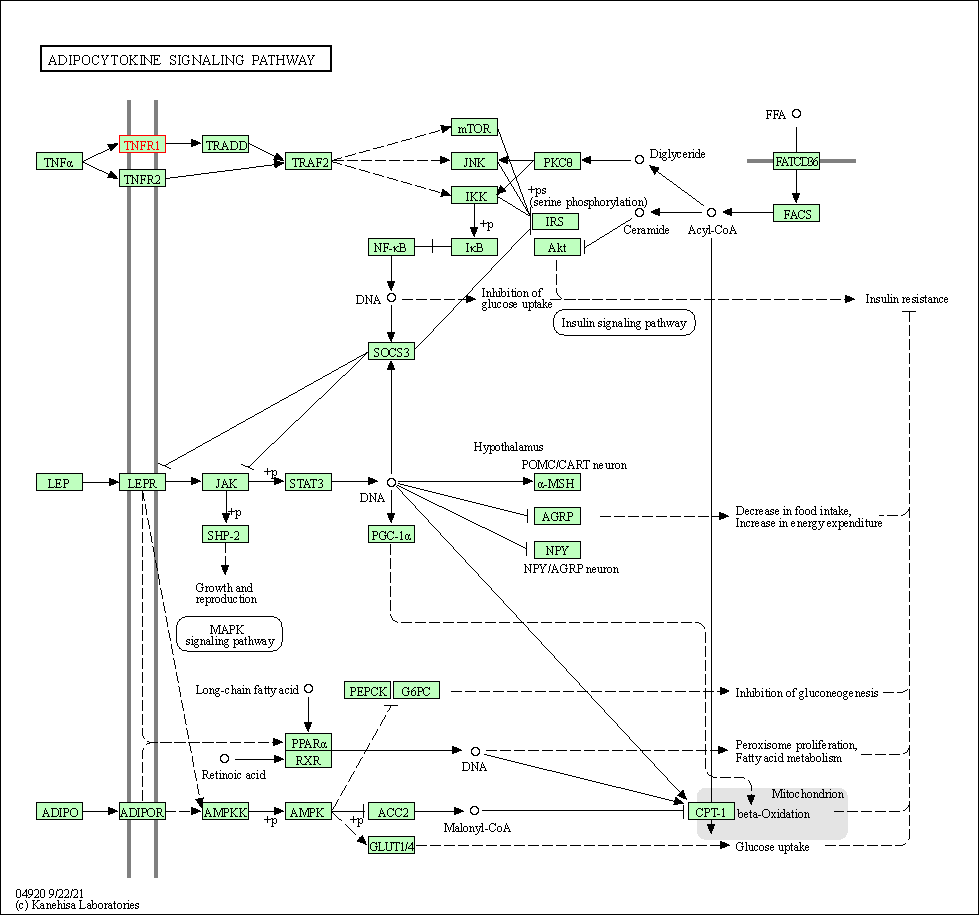
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Sphingolipid signaling pathway | hsa04071 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| mTOR signaling pathway | hsa04150 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Apoptosis - multiple species | hsa04215 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Necroptosis | hsa04217 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Adipocytokine signaling pathway | hsa04920 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 49 | Degree centrality | 5.26E-03 | Betweenness centrality | 3.59E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.49E-01 | Radiality | 1.43E+01 | Clustering coefficient | 1.96E-01 |
| Neighborhood connectivity | 3.15E+01 | Topological coefficient | 5.06E-02 | Eccentricity | 10 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Phase I dose-escalation study of VB-111, an antiangiogenic virotherapy, in patients with advanced solid tumors.Clin Cancer Res.2013 Jul 15;19(14):3996-4007. | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | ClinicalTrials.gov (NCT02221037) Study of GSK2862277 in Subjects Undergoing Oesophagectomy Surgery. U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT01759056) Evaluation of an Oral Anti-TNF Antibody in Patients With Active Ulcerative Colitis. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT01587807) A Study to Investigate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Doses of Inhaled GSK1995057. U.S. National Institutes of Health. | |||||
| REF 6 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007694) | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1870). | |||||
| REF 8 | Secretion of a TNFR:Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J Virol. 2004 Nov;78(22):12355-65. | |||||
| REF 9 | Autoantibodies to variable heavy (VH) chain Ig sequences in humans impact the safety and clinical pharmacology of a VH domain antibody antagonist of TNF-alpha receptor 1. J Clin Immunol. 2013 Oct;33(7):1192-203. | |||||
| REF 10 | Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-alpha. Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):11879-84. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

