Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T89988
(Former ID: TTDC00122)
|
|||||
| Target Name |
C-C chemokine receptor type 2 (CCR2)
|
|||||
| Synonyms |
Monocyte chemoattractant protein 1 receptor; MCP-1-R; Chemokine receptor CCR2B; CMKBR2; CD192; CCR-2; CC-CKR-2; C-C CKR-2
Click to Show/Hide
|
|||||
| Gene Name |
CCR2
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 10 Target-related Diseases | + | ||||
| 1 | Chronic kidney disease [ICD-11: GB61] | |||||
| 2 | Chronic obstructive pulmonary disease [ICD-11: CA22] | |||||
| 3 | Human immunodeficiency virus disease [ICD-11: 1C60-1C62] | |||||
| 4 | Metastatic tumour [ICD-11: 2D50-2E2Z] | |||||
| 5 | Multiple sclerosis [ICD-11: 8A40] | |||||
| 6 | Pancreatic cancer [ICD-11: 2C10] | |||||
| 7 | Chronic pain [ICD-11: MG30] | |||||
| 8 | Melanoma [ICD-11: 2C30] | |||||
| 9 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| 10 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Its binding with CCL2 on monocytes and macrophages mediates chemotaxis and migration induction through the activation of the PI3K cascade, the small G protein Rac and lamellipodium protrusion. Also acts as a receptor for the beta-defensin DEFB106A/DEFB106B. Regulates the expression of T-cell inflammatory cytokines and T-cell differentiation, promoting the differentiation of T-cells into T-helper 17 cells (Th17) during inflammation. Faciltates the export of mature thymocytes by enhancing directional movement of thymocytes to sphingosine-1-phosphate stimulation and up-regulation of S1P1R expression; signals through the JAK-STAT pathway to regulate FOXO1 activity leading to an increased expression of S1P1R. Plays an important role in mediating peripheral nerve injury-induced neuropathic pain. Increases NMDA-mediated synaptic transmission in both dopamine D1 and D2 receptor-containing neurons, which may be caused by MAPK/ERK-dependent phosphorylation of GRIN2B/NMDAR2B. Mediates the recruitment of macrophages and monocytes to the injury site following brain injury. Key functional receptor for CCL2 but can also bind CCL7 and CCL12.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MLSTSRSRFIRNTNESGEEVTTFFDYDYGAPCHKFDVKQIGAQLLPPLYSLVFIFGFVGN
MLVVLILINCKKLKCLTDIYLLNLAISDLLFLITLPLWAHSAANEWVFGNAMCKLFTGLY HIGYFGGIFFIILLTIDRYLAIVHAVFALKARTVTFGVVTSVITWLVAVFASVPGIIFTK CQKEDSVYVCGPYFPRGWNNFHTIMRNILGLVLPLLIMVICYSGILKTLLRCRNEKKRHR AVRVIFTIMIVYFLFWTPYNIVILLNTFQEFFGLSNCESTSQLDQATQVTETLGMTHCCI NPIIYAFVGEKFRSLFHIALGCRIAPLQKPVCGGPGVRPGKNVKVTTQGLLDGRGKGKSI GRAPEASLQDKEGA Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T20W1O | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | AZD-2423 | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [2] | |
| 2 | BMS-813160 | Drug Info | Phase 2 | Diabetic nephropathy | [3] | |
| 3 | CCX-140 | Drug Info | Phase 2 | Diabetic nephropathy | [4] | |
| 4 | CCX872 | Drug Info | Phase 2 | Pancreatic tumour | [5] | |
| 5 | Cenicriviroc | Drug Info | Phase 2 | Human immunodeficiency virus infection | [6], [7] | |
| 6 | MK-0812 | Drug Info | Phase 2 | Multiple sclerosis | [8] | |
| 7 | MLN1202 | Drug Info | Phase 2 | Multiple sclerosis | [9] | |
| 8 | Aminoguanidine | Drug Info | Phase 1 | Diabetic retinopathy | [10] | |
| 9 | CNTX-6970 | Drug Info | Phase 1 | Inflammatory pain | [11] | |
| 10 | OPL-CCL2-LPM | Drug Info | Phase 1 | Arthritis | [12] | |
| 11 | TAK-202 | Drug Info | Phase 1 | Melanoma | [13] | |
| Discontinued Drug(s) | [+] 5 Discontinued Drugs | + | ||||
| 1 | CCX915 | Drug Info | Discontinued in Phase 1 | Multiple sclerosis | [14] | |
| 2 | INCB8696 | Drug Info | Discontinued in Phase 1 | Multiple sclerosis | [15] | |
| 3 | AZD-6942 | Drug Info | Terminated | Rheumatoid arthritis | [18] | |
| 4 | INCB3344 | Drug Info | Terminated | Arteriosclerosis | [19], [20] | |
| 5 | SB-282241 | Drug Info | Terminated | Inflammation | [21], [22] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | MCP-1 | Drug Info | Preclinical | Rheumatoid arthritis | [16] | |
| 2 | RS-504393 | Drug Info | Preclinical | Chronic obstructive pulmonary disease | [16], [17] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Antagonist | [+] 22 Antagonist drugs | + | ||||
| 1 | AZD-2423 | Drug Info | [23] | |||
| 2 | CCX-140 | Drug Info | [1] | |||
| 3 | Cenicriviroc | Drug Info | [27] | |||
| 4 | MK-0812 | Drug Info | [28] | |||
| 5 | MLN1202 | Drug Info | [16] | |||
| 6 | Aminoguanidine | Drug Info | [29] | |||
| 7 | CNTX-6970 | Drug Info | [11] | |||
| 8 | TAK-202 | Drug Info | [5], [13] | |||
| 9 | CCX915 | Drug Info | [31] | |||
| 10 | INCB8696 | Drug Info | [32] | |||
| 11 | MCP-1 | Drug Info | [16] | |||
| 12 | RS-504393 | Drug Info | [16], [33] | |||
| 13 | AZD-6942 | Drug Info | [34] | |||
| 14 | INCB3344 | Drug Info | [35] | |||
| 15 | SB-282241 | Drug Info | [34] | |||
| 16 | BMS-A | Drug Info | [37] | |||
| 17 | GSK-1344386B | Drug Info | [37] | |||
| 18 | INCB-10820 | Drug Info | [37] | |||
| 19 | RS-102895 | Drug Info | [39] | |||
| 20 | RS-136270 | Drug Info | [39] | |||
| 21 | Teijin-lead_cmp_5 | Drug Info | [22] | |||
| 22 | viral macrophage inflammatory protein-II | Drug Info | [40] | |||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | BMS-813160 | Drug Info | [24], [25] | |||
| 2 | OPL-CCL2-LPM | Drug Info | [30] | |||
| 3 | RAP-103 | Drug Info | [37] | |||
| 4 | RAP-310 | Drug Info | [37] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | CCX872 | Drug Info | [26] | |||
| 2 | (R)-3-(1'-Adamantanecarbonyl)amino-caprolactam | Drug Info | [36] | |||
| 3 | (S)-3-(1'-Adamantanecarbonyl)amino-caprolactam | Drug Info | [36] | |||
| 4 | (S)-3-(2',2'-Dimethyl-propionyl)amino-caprolactam | Drug Info | [36] | |||
| 5 | NSC-651016 | Drug Info | [38] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: (3s)-1-{(1s,2r,4r)-4-[methyl(Propan-2-Yl)amino]-2-Propylcyclohexyl}-3-{[6-(Trifluoromethyl)quinazolin-4-Yl]amino}pyrrolidin-2-One | Ligand Info | |||||
| Structure Description | Structure of CC Chemokine Receptor 2 with Orthosteric and Allosteric Antagonists | PDB:5T1A | ||||
| Method | X-ray diffraction | Resolution | 2.81 Å | Mutation | Yes | [41] |
| PDB Sequence |
VKQIGAQLLP
46 PLYSLVFIFG56 FVGNMLVVLI66 LINCKKLKCL76 TDIYLLNLAI86 SDLLFLITLP 96 LWAHSAANEW106 VFGNAMCKLF116 TGLYHIGYFG126 GIFFIILLTI136 DRYLAIVHAV 146 FALKARTVTF156 GVVTSVITWL166 VAVFASVPGI176 IFTKQKEDSV187 YVCGPYFPRG 197 WNNFHTIMRN207 ILGLVLPLLI217 MVICYSGISR227 ASKSRINIFE1005 MLRIDEGLRL 1015 KIYKDTEGYY1025 TIGIGHLLTK1035 SPSLNAAKSE1045 LDKAIGRNTN1055 GVITKDEAEK 1065 LFNQDVDAAV1075 RGILRNAKLK1085 PVYDSLDAVR1095 RAALINMVFQ1105 MGETGVAGFT 1115 NSLRMLQQKR1125 WDEAAVNLAK1135 SRWYNQTPNR1145 AKRVITTFRT1155 GTWDAYPPPS 236 REKKAVRVIF246 TIMIVYFLFW256 TPYNIVILLN266 TFQEFFGLSN276 CESTSQLDQA 286 TQVTETLGMT296 HCCINPIIYA306 FVGEKFRRYL316 SVFF
|
|||||
|
|
||||||
| Ligand Name: 2H-Pyrrol-2-one, 4-acetyl-1-(4-chloro-2-fluorophenyl)-5-cyclohexyl-1,5-dihydro-3-hydroxy-, (5R)- | Ligand Info | |||||
| Structure Description | Structure of CC Chemokine Receptor 2 with Orthosteric and Allosteric Antagonists | PDB:5T1A | ||||
| Method | X-ray diffraction | Resolution | 2.81 Å | Mutation | Yes | [41] |
| PDB Sequence |
VKQIGAQLLP
46 PLYSLVFIFG56 FVGNMLVVLI66 LINCKKLKCL76 TDIYLLNLAI86 SDLLFLITLP 96 LWAHSAANEW106 VFGNAMCKLF116 TGLYHIGYFG126 GIFFIILLTI136 DRYLAIVHAV 146 FALKARTVTF156 GVVTSVITWL166 VAVFASVPGI176 IFTKQKEDSV187 YVCGPYFPRG 197 WNNFHTIMRN207 ILGLVLPLLI217 MVICYSGISR227 ASKSRINIFE1005 MLRIDEGLRL 1015 KIYKDTEGYY1025 TIGIGHLLTK1035 SPSLNAAKSE1045 LDKAIGRNTN1055 GVITKDEAEK 1065 LFNQDVDAAV1075 RGILRNAKLK1085 PVYDSLDAVR1095 RAALINMVFQ1105 MGETGVAGFT 1115 NSLRMLQQKR1125 WDEAAVNLAK1135 SRWYNQTPNR1145 AKRVITTFRT1155 GTWDAYPPPS 236 REKKAVRVIF246 TIMIVYFLFW256 TPYNIVILLN266 TFQEFFGLSN276 CESTSQLDQA 286 TQVTETLGMT296 HCCINPIIYA306 FVGEKFRRYL316 SVFF
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
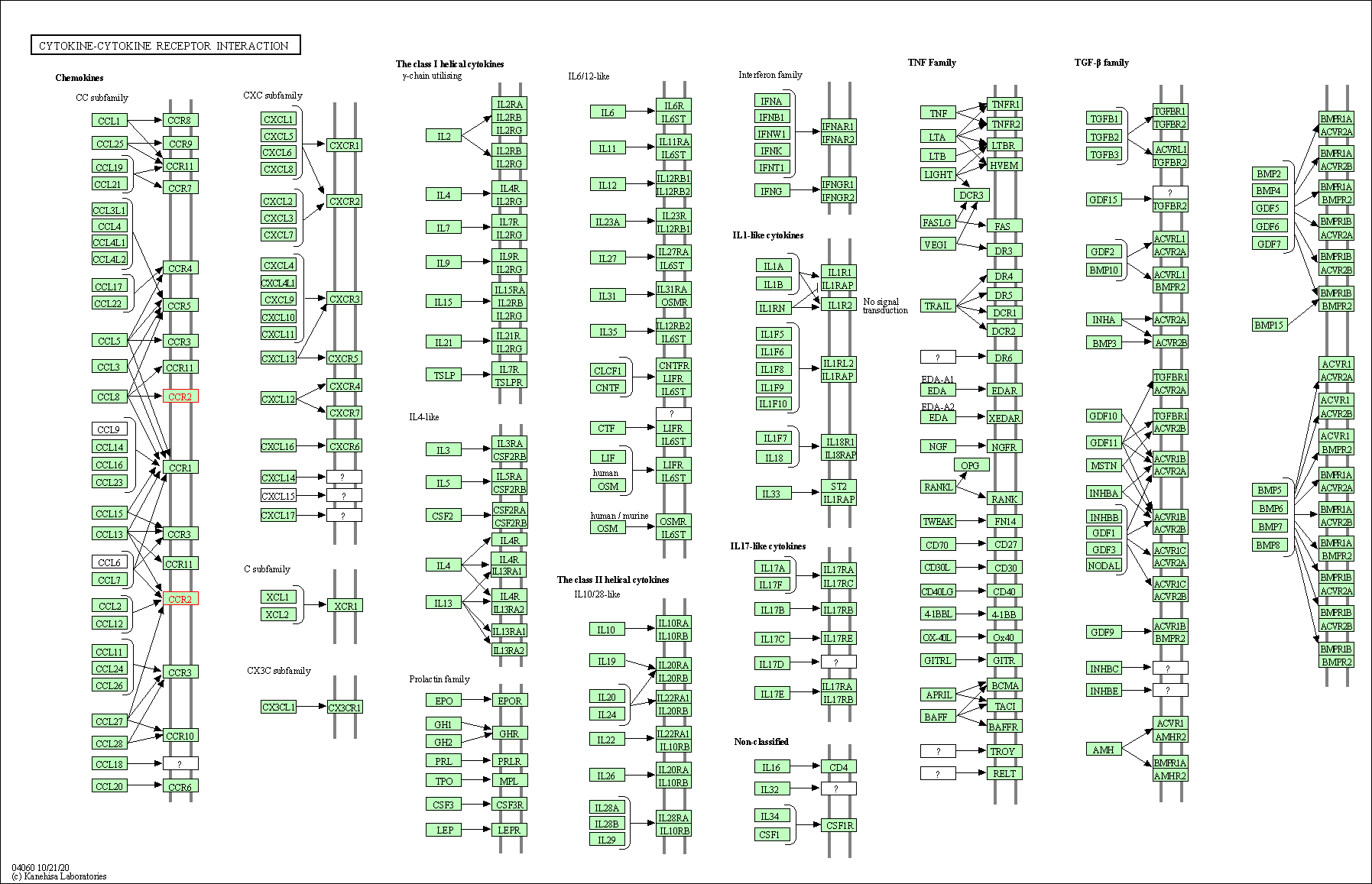
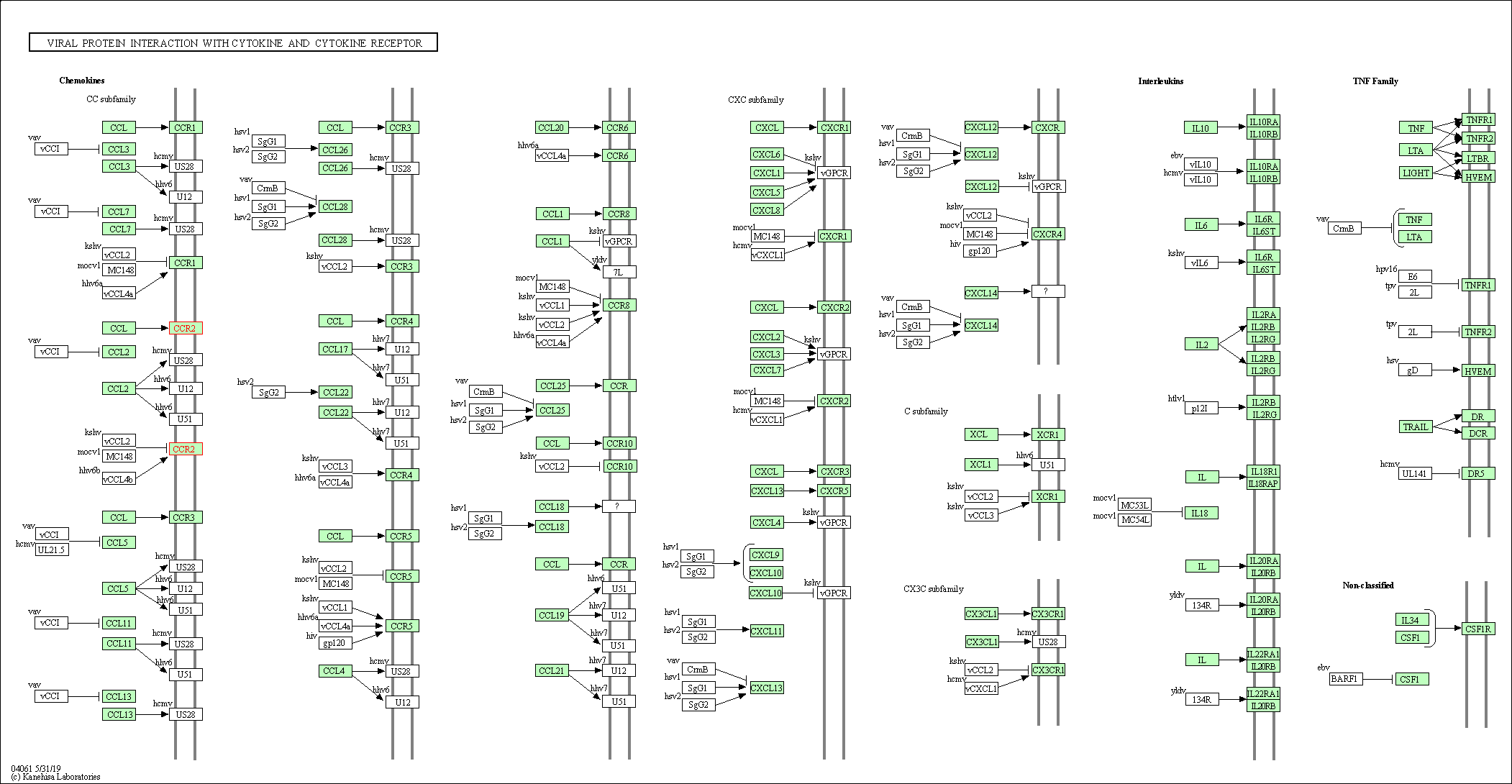
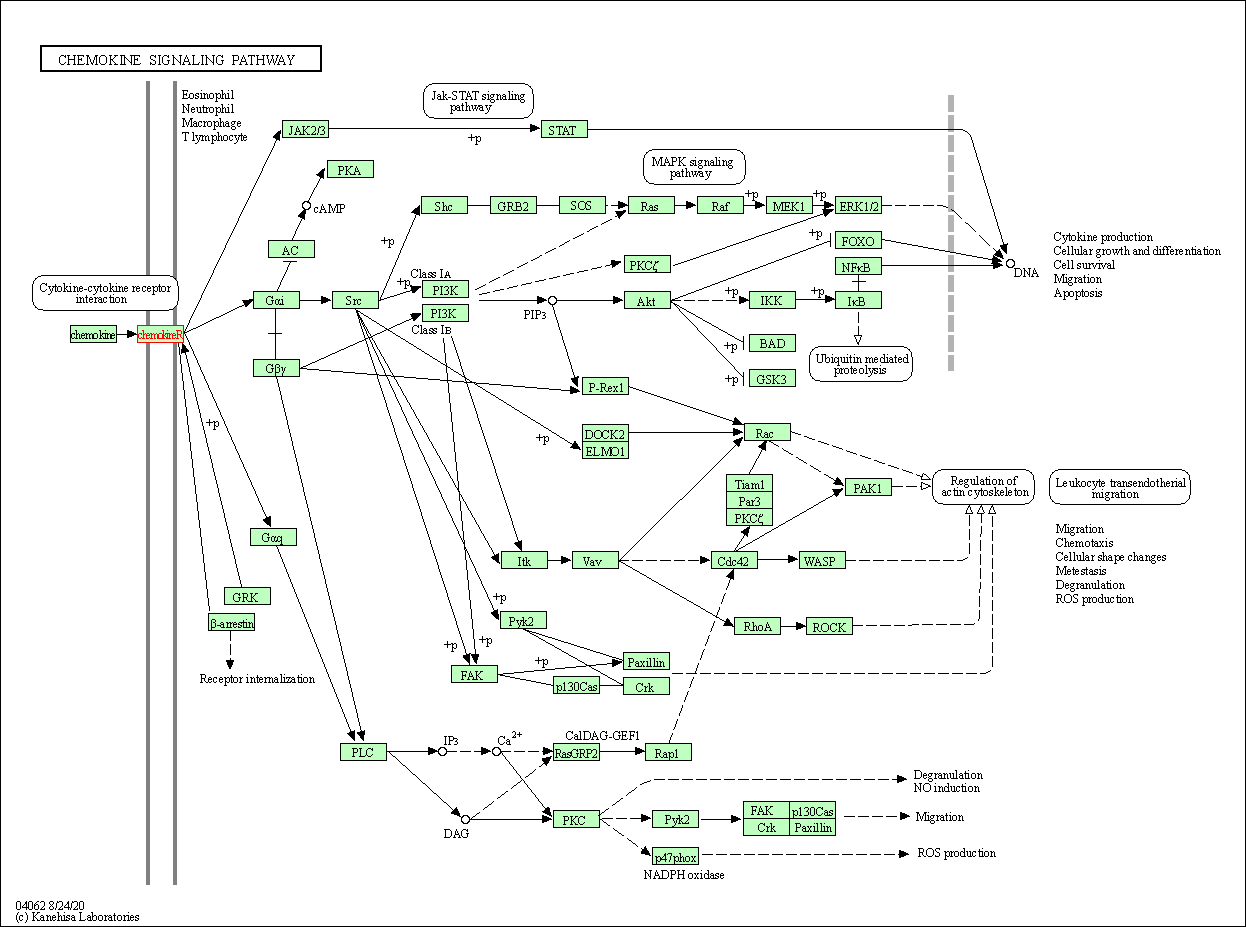
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Chemokine signaling pathway | hsa04062 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 11 | Degree centrality | 1.18E-03 | Betweenness centrality | 4.33E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.11E-01 | Radiality | 1.37E+01 | Clustering coefficient | 1.09E-01 |
| Neighborhood connectivity | 2.03E+01 | Topological coefficient | 1.51E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | Chemokine signaling pathway | |||||
| NetPath Pathway | [+] 5 NetPath Pathways | + | ||||
| 1 | TCR Signaling Pathway | |||||
| 2 | IL2 Signaling Pathway | |||||
| 3 | TGF_beta_Receptor Signaling Pathway | |||||
| 4 | Leptin Signaling Pathway | |||||
| 5 | RANKL Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Inflammation mediated by chemokine and cytokine signaling pathway | |||||
| Reactome | [+] 3 Reactome Pathways | + | ||||
| 1 | Beta defensins | |||||
| 2 | Chemokine receptors bind chemokines | |||||
| 3 | G alpha (i) signalling events | |||||
| WikiPathways | [+] 6 WikiPathways | + | ||||
| 1 | GPCRs, Class A Rhodopsin-like | |||||
| 2 | Defensins | |||||
| 3 | Spinal Cord Injury | |||||
| 4 | Peptide GPCRs | |||||
| 5 | GPCR ligand binding | |||||
| 6 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Present and future in the treatment of diabetic kidney disease. J Diabetes Res. 2015;2015:801348. | |||||
| REF 2 | ClinicalTrials.gov (NCT01215279) AZD2423 Safety and Tolerability Study in Patients With Moderate and Severe Chronic Obstructive Pulmonary Disease(COPD) in AstraZeneca. | |||||
| REF 3 | ClinicalTrials.gov (NCT01752985) Study to Evaluate the Effects of BMS-813160 on Protein Loss in the Urine of Subjects With Type 2 Diabetes and Diabetic Kidney Disease. U.S. National Institutes of Health. | |||||
| REF 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018498) | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 801). | |||||
| REF 7 | The dual CCR5 and CCR2 inhibitor cenicriviroc does not redistribute HIV into extracellular space: implications for plasma viral load and intracellular DNA decline. J Antimicrob Chemother. 2015 Mar;70(3):750-6. | |||||
| REF 8 | ClinicalTrials.gov (NCT00239655) A Study to Assess the Effects of MK0812 on Disease Activity in Patients With Relapsing-Remitting Multiple Sclerosis as Measured by Magnetic Resonance Imaging (MRI). U.S. National Institutes of Health. | |||||
| REF 9 | Emerging drugs for the therapy of primary and post essential thrombocythemia, post polycythemia vera myelofibrosis. Expert Opin Emerg Drugs. 2009 Sep;14(3):471-9. | |||||
| REF 10 | ClinicalTrials.gov (NCT02099981) Restoration of Retinal Vascular Responses in Type 1 Diabetic Patients in University of Minnesota - Clinical and Translational Science Institute. | |||||
| REF 11 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800030092) | |||||
| REF 13 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035649) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024340) | |||||
| REF 16 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | |||||
| REF 17 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 781). | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016328) | |||||
| REF 19 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 777). | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035662) | |||||
| REF 21 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 782). | |||||
| REF 22 | CCR2: characterization of the antagonist binding site from a combined receptor modeling/mutagenesis approach. J Med Chem. 2003 Sep 11;46(19):4070-86. | |||||
| REF 23 | CA patent application no. 841416, Method of selecting therapeutic indications. | |||||
| REF 24 | A dual CCR2/CCR5 chemokine antagonist, BMS-813160. Expert Opin Ther Pat. 2011 Dec;21(12):1919-24. | |||||
| REF 25 | Methods for improving thymic recovery and preventing and treating graft versus host disease using ccr2 and ccr5 antagonists | |||||
| REF 26 | Therapeutic use of a clinical stage CCR2 inhibitor, CCX872, in obesity-associated steatohepatitis. The Lancet Volume 383, Supplement 1, 26 February 2014, Pages S78. | |||||
| REF 27 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 28 | Assessment of chemokine receptor function on monocytes in whole blood: In vitro and ex vivo evaluations of a CCR2 antagonist. J Immunol Methods. 2010 Jan 31;352(1-2):101-10. | |||||
| REF 29 | Incyte Announces Second Quarter 2005 Financial Results; Reports Positive Phase IIb Results for Reverset in Treatment-Experienced HIV Patients. Incyte Corporation. 2005. | |||||
| REF 30 | Selective CCR2-targeted macrophage depletion ameliorates experimental mesangioproliferative glomerulonephritis. Clin Exp Immunol. 2009 February; 155(2): 295-303. | |||||
| REF 31 | Marketed and experimental medicines for the treatment of multiple sclerosis. The Association of the British Pharmaceutical Industry. 2009. | |||||
| REF 32 | Incyte. Product Development Pipeline. | |||||
| REF 33 | Beneficial or detrimental effects of carotenoids contained in food: cell culture models. Mini Rev Med Chem. 2007 Nov;7(11):1120-8. | |||||
| REF 34 | Patent EP2727944 A1. | |||||
| REF 35 | Discovery of ((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-4-ylamino)cyclopentyl)(4-(5-(trifluoromethyl)pyridazin-3-yl)piperazin-1-yl)methanone, PF-4254196, a CCR2 antagonist with an improved cardiovascular profile. Bioorg Med Chem Lett. 2011 May 1;21(9):2626-30. | |||||
| REF 36 | Highly potent, orally available anti-inflammatory broad-spectrum chemokine inhibitors. J Med Chem. 2009 Jun 11;52(11):3591-5. | |||||
| REF 37 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 59). | |||||
| REF 38 | Inhibition of in vitro and in vivo HIV replication by a distamycin analogue that interferes with chemokine receptor function: a candidate for chemo... J Med Chem. 1998 Jun 18;41(13):2184-93. | |||||
| REF 39 | Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within t... J Biol Chem. 2000 Aug 18;275(33):25562-71. | |||||
| REF 40 | A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997 Sep 12;277(5332):1656-9. | |||||
| REF 41 | Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature. 2016 Dec 15;540(7633):458-461. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

