Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T99582
|
|||||
| Target Name |
HUMAN interleukin 6 (IL6)
|
|||||
| Synonyms |
Interferon beta-2; IL-6; IFNB2; IFN-beta-2; Hybridoma growth factor; CTL differentiation factor; CDF; BSF-2; B-cell stimulatory factor 2
Click to Show/Hide
|
|||||
| Gene Name |
IL6
|
|||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Idiopathic multicentric Castlemans disease [ICD-11: 2A81] | |||||
| Function |
It is a potent inducer of the acute phase response. Plays an essential role in the final differentiation of B-cells into Ig-secreting cells Involved in lymphocyte and monocyte differentiation. Acts on B-cells, T-cells, hepatocytes, hematopoietic progenitor cells and cells of the CNS. Required for the generation of T(H)17 cells. Also acts as a myokine. It is discharged into the bloodstream after muscle contraction and acts to increase the breakdown of fats and to improve insulin resistance. It induces myeloma and plasmacytoma growth and induces nerve cells differentiation. Cytokine with a wide variety of biological functions.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MNSFSTSAFGPVAFSLGLLLVLPAAFPAPVPPGEDSKDVAAPHRQPLTSSERIDKQIRYI
LDGISALRKETCNKSNMCESSKEALAENNLNLPKMAEKDGCFQSGFNEETCLVKIITGLL EFEVYLEYLQNRFESSEEQARAVQMSTKVLIQFLQKKAKNLDAITTPDPTTNASLLTKLQ AQNQWLQDMTTHLILRSFKEFLQSSLRALRQM Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Drugs in Phase 3 Trial | [+] 1 | + | ||||
| 1 | Siltuximab | Drug Info | Approved | Idiopathic multicentric Castlemans disease | [2] | |
| Drugs in Phase 2 Trial | [+] 3 | + | ||||
| 1 | Olokizumab | Drug Info | Phase 3 | Rheumatoid arthritis | [3] | |
| 2 | Sirukumab | Drug Info | Phase 3 | Rheumatoid arthritis | [4] | |
| 3 | Clazakizumab | Drug Info | Phase 2 | Coronavirus Disease 2019 (COVID-19) | [5] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | Siltuximab | Drug Info | [6], [7] | |||
| 2 | Olokizumab | Drug Info | [7], [8] | |||
| 3 | Clazakizumab | Drug Info | [7], [9] | |||
| 4 | Sirukumab | Drug Info | [7], [10] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| FoxO signaling pathway | hsa04068 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cellular senescence | hsa04218 | Affiliated Target |
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| NOD-like receptor signaling pathway | hsa04621 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Cytosolic DNA-sensing pathway | hsa04623 | Affiliated Target |
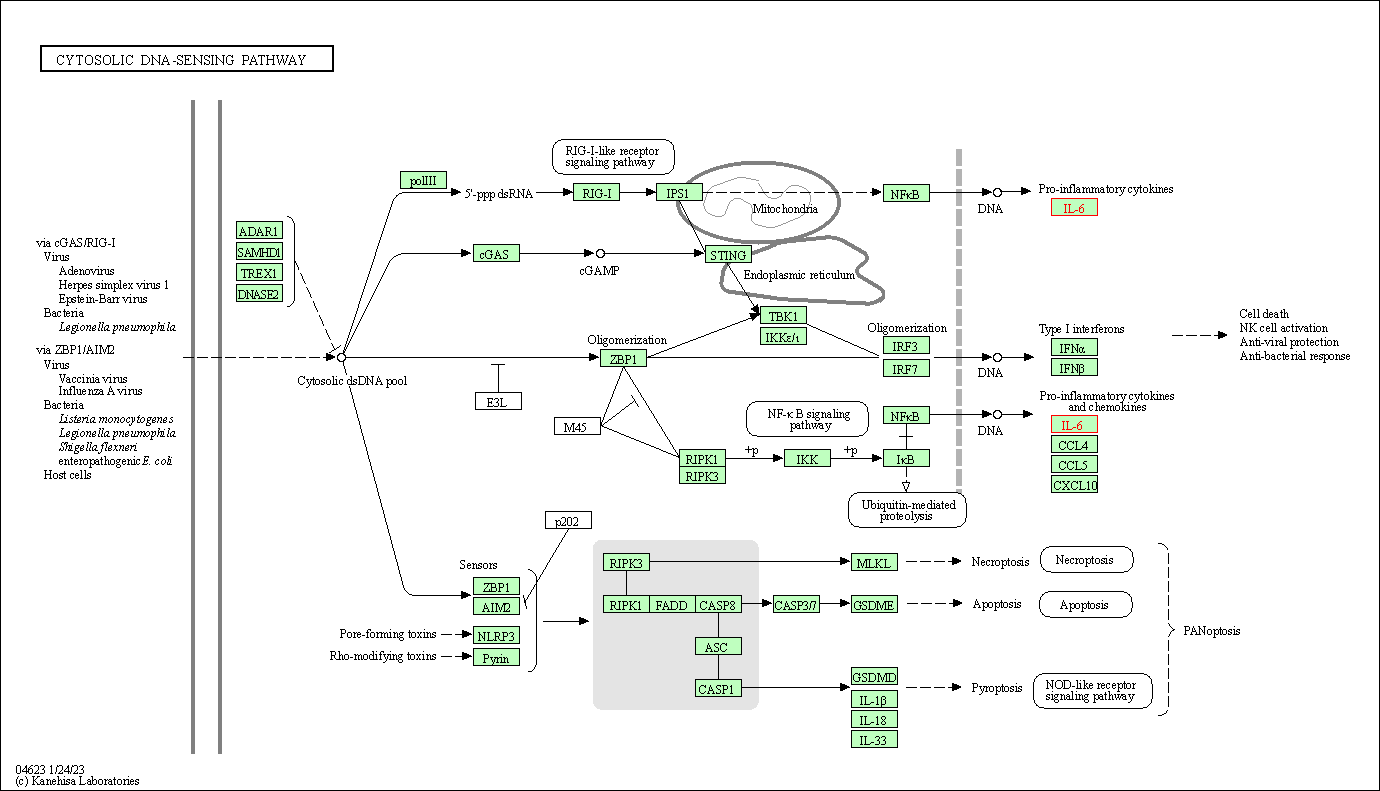
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |
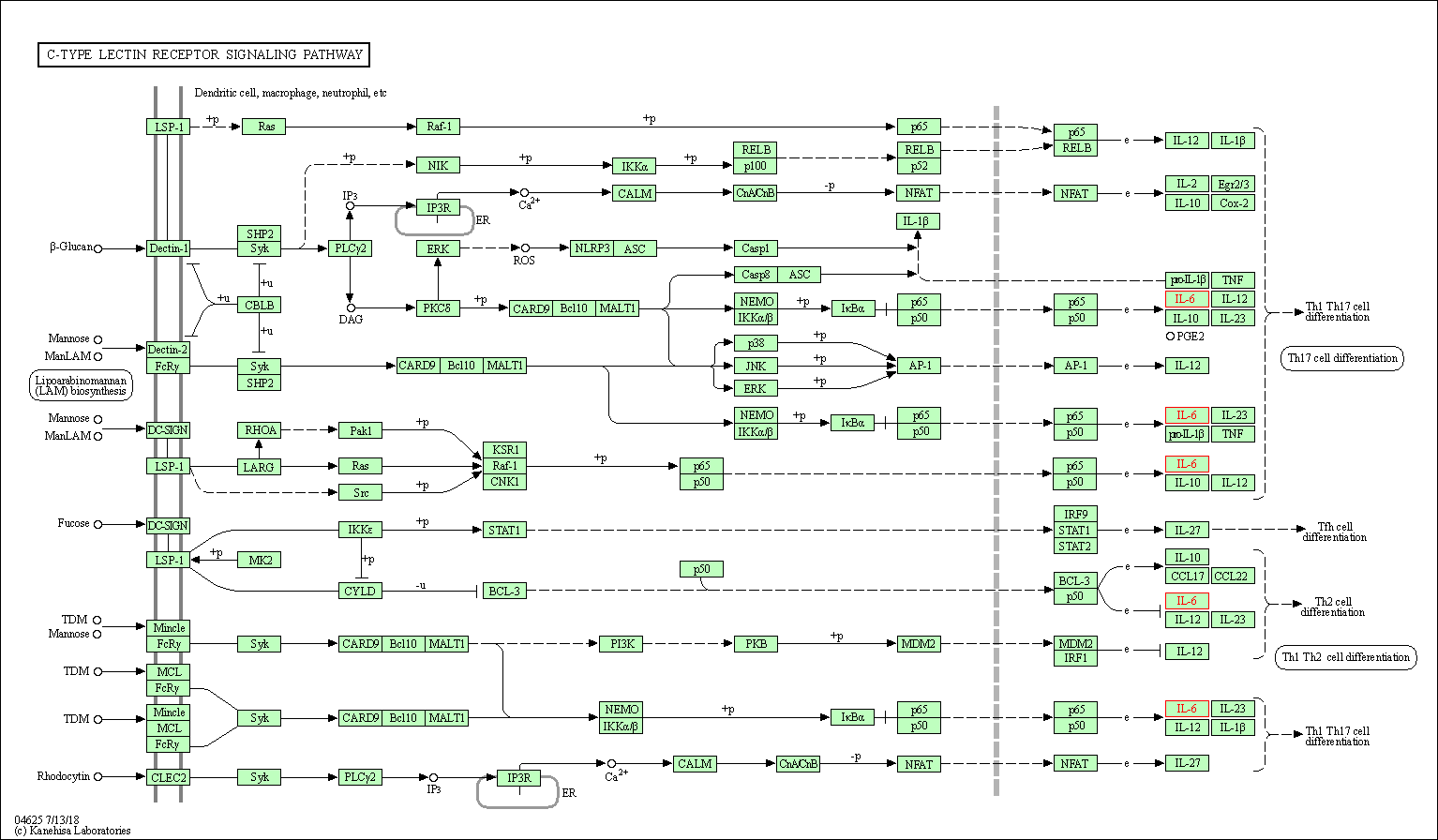
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
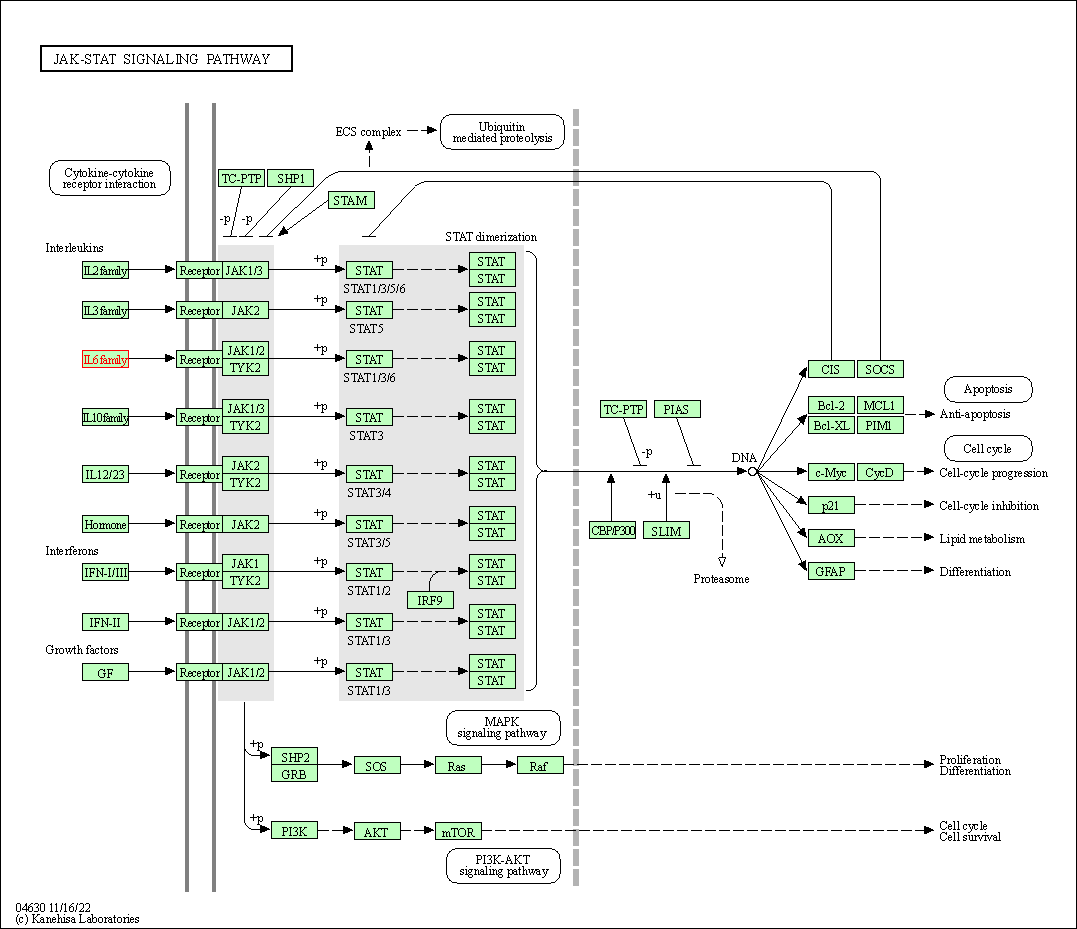
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
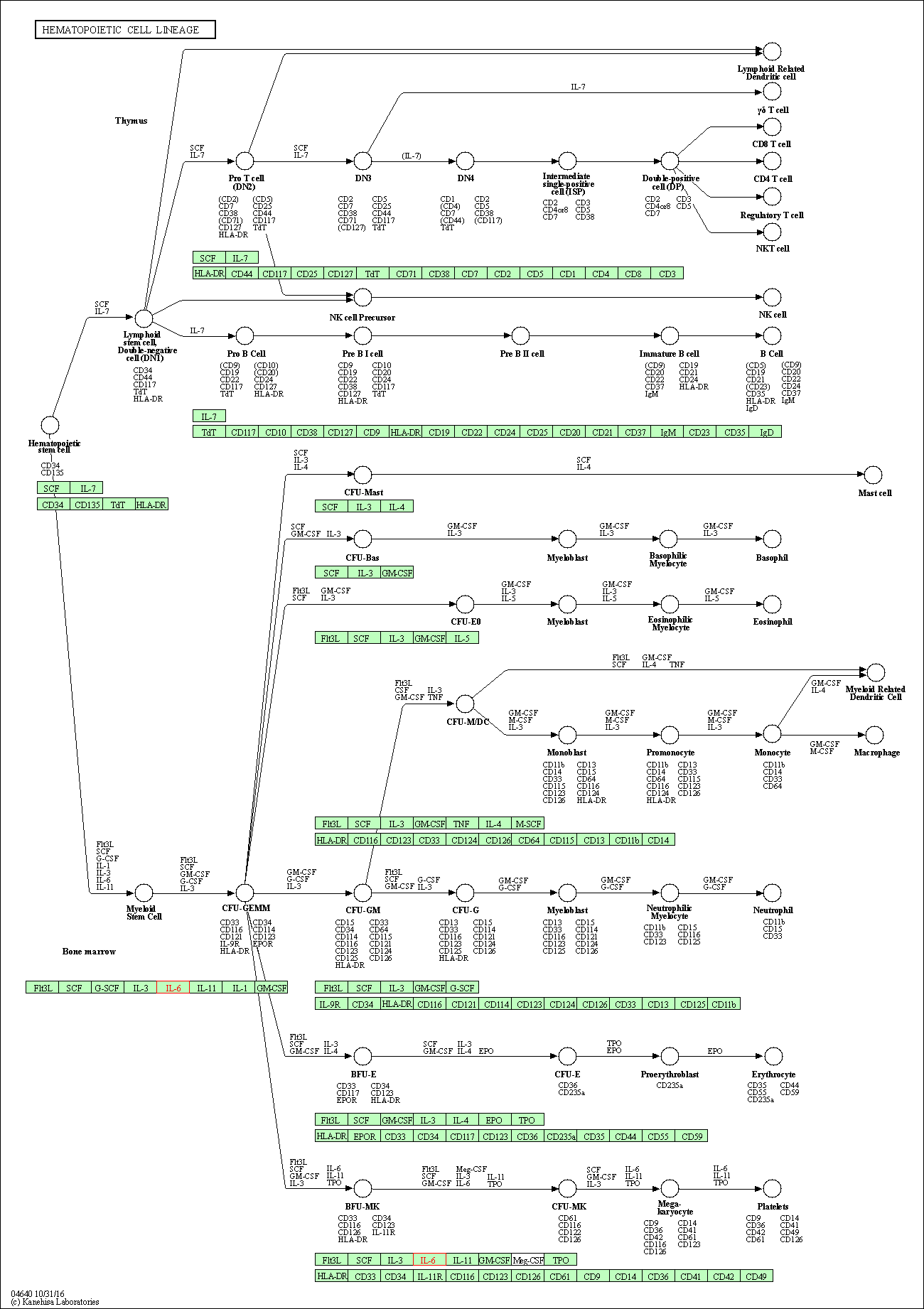
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| IL-17 signaling pathway | hsa04657 | Affiliated Target |
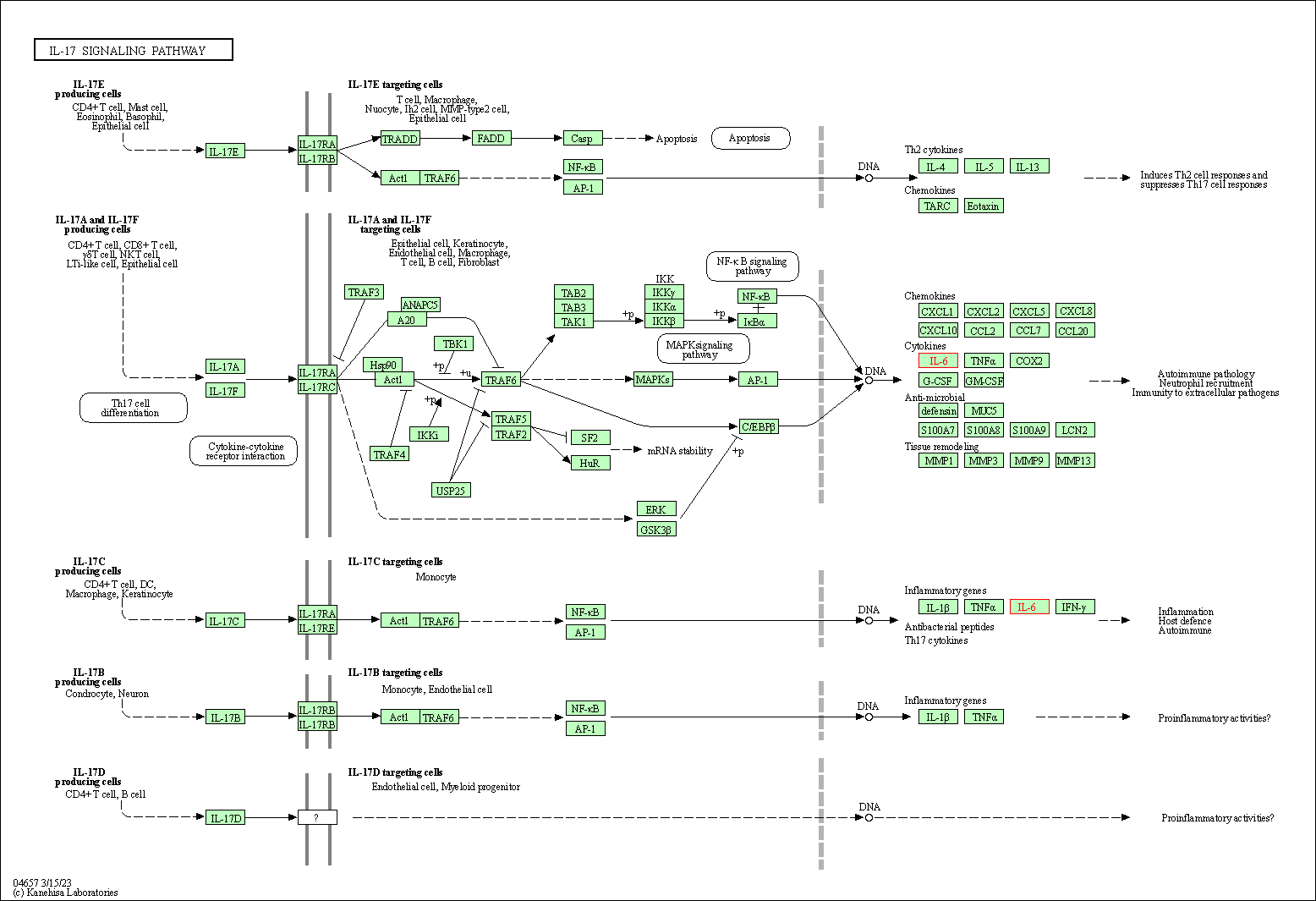
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Th17 cell differentiation | hsa04659 | Affiliated Target |
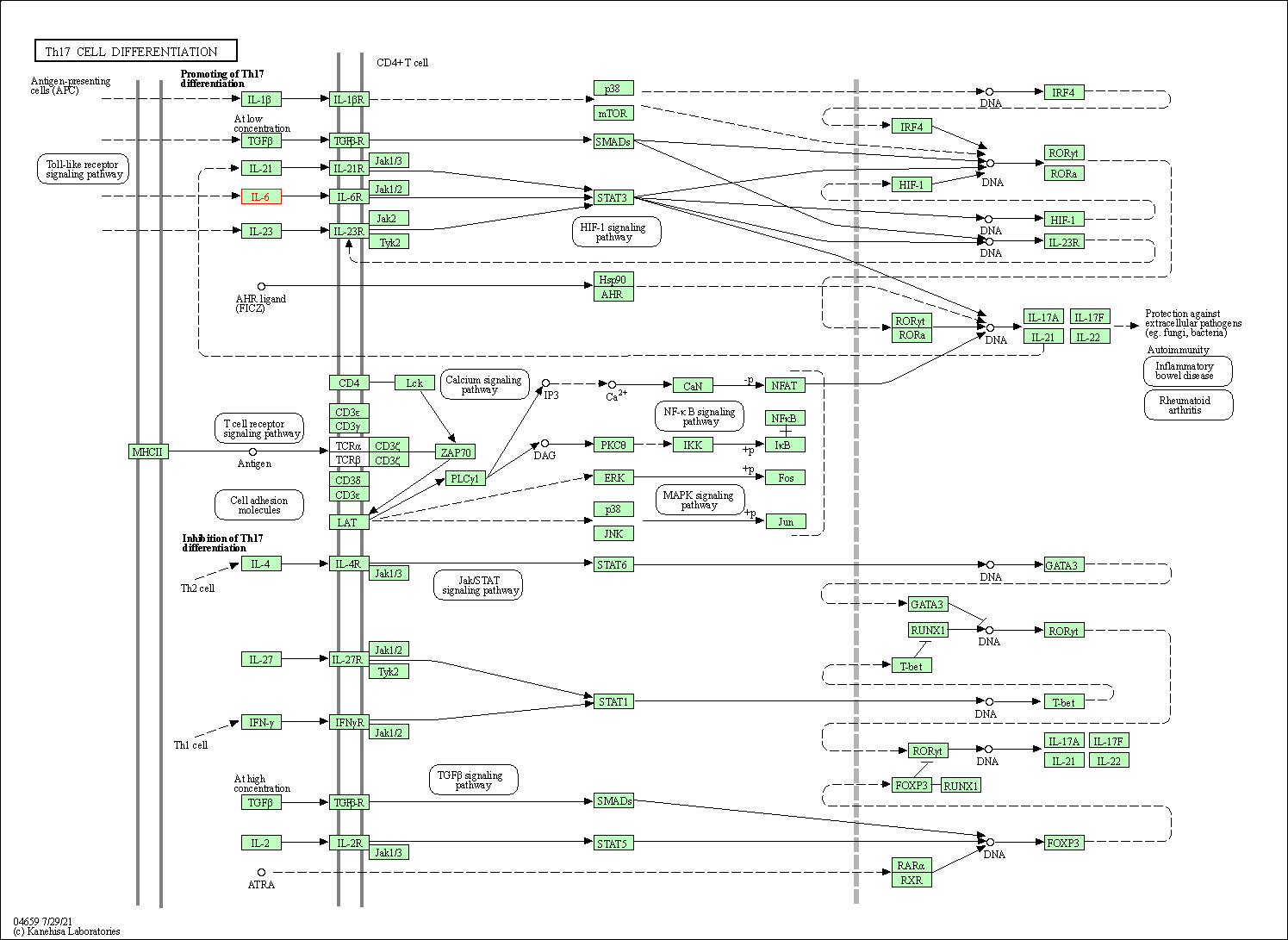
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |
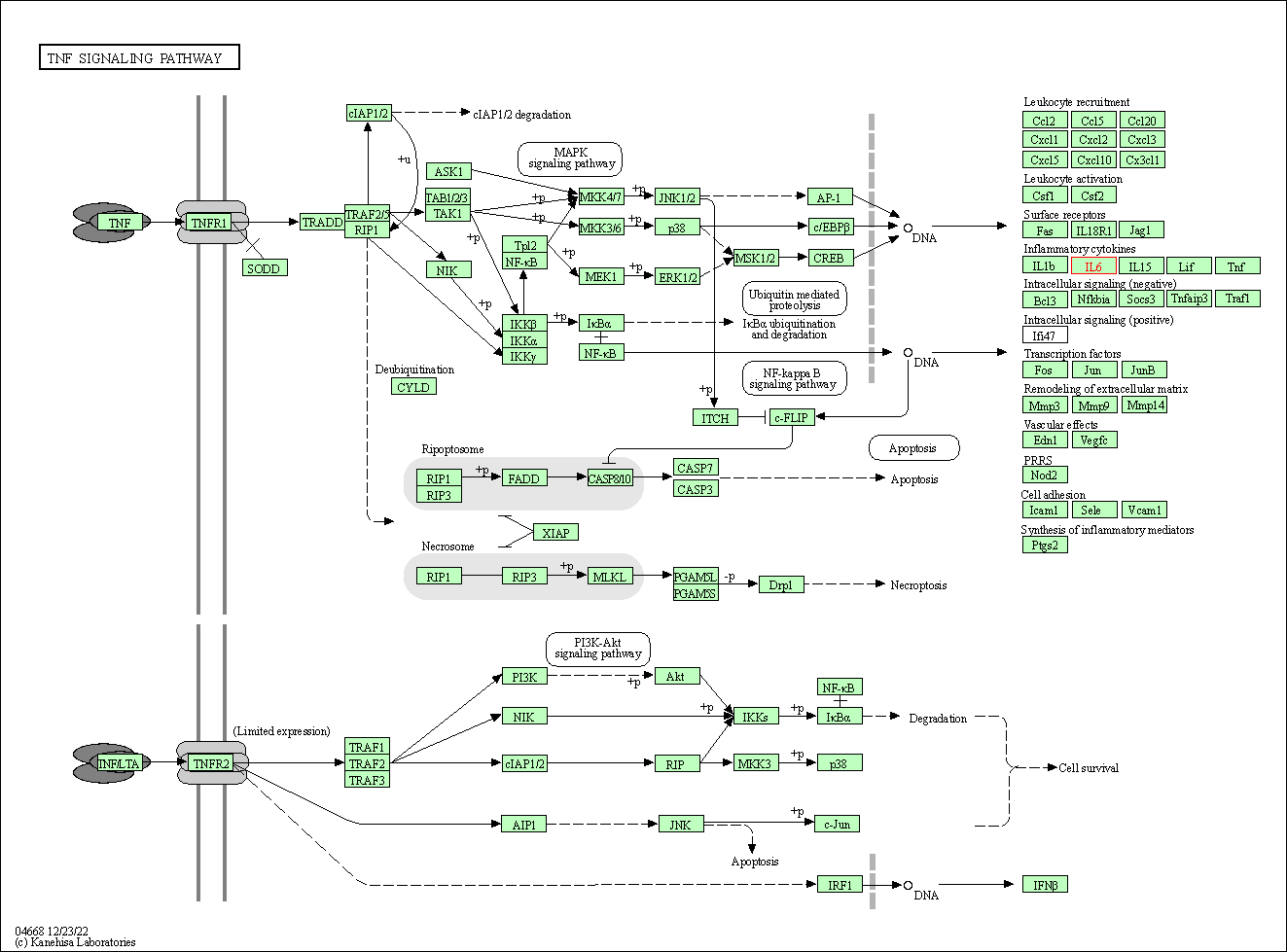
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |
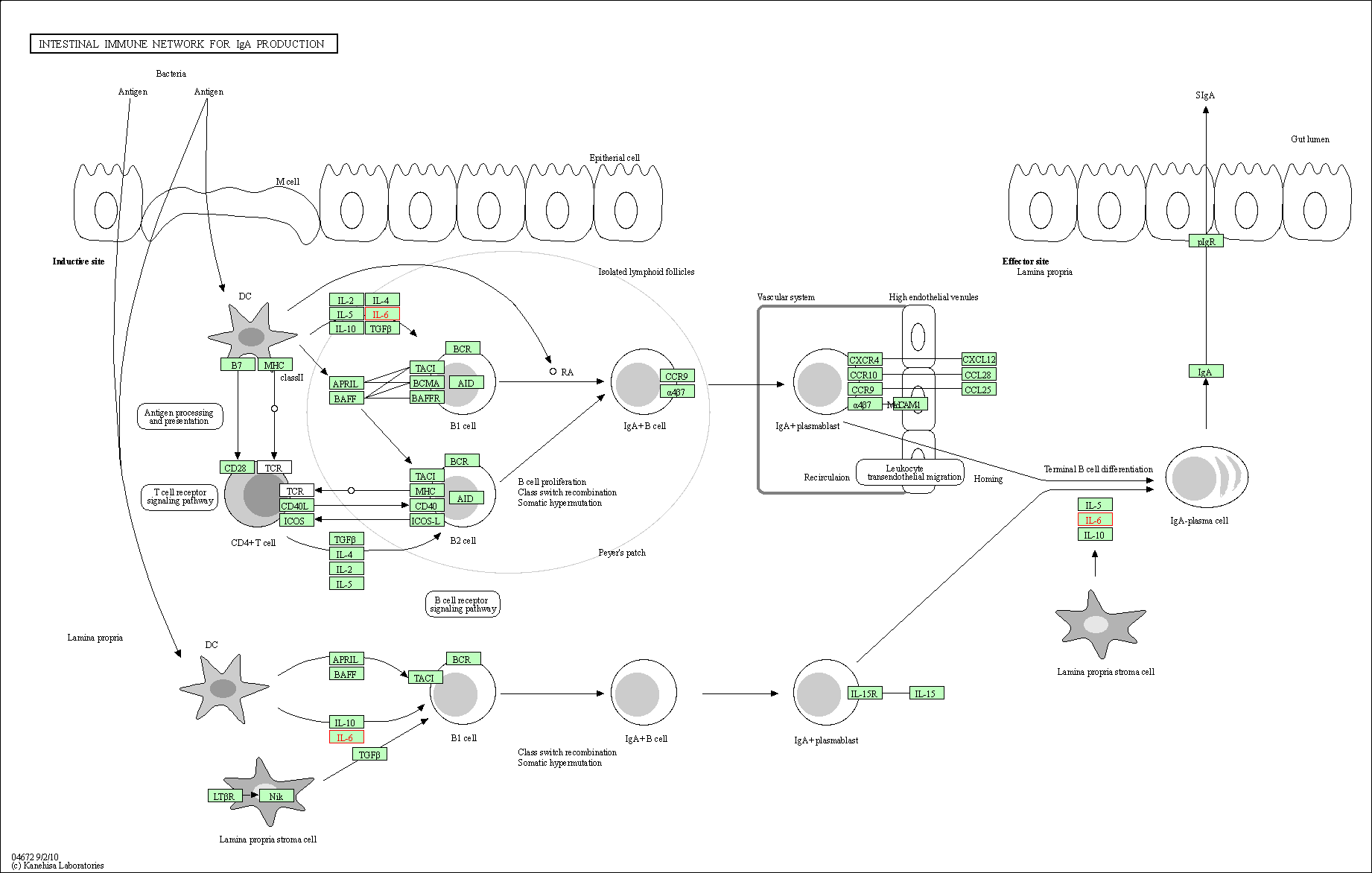
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 62 | Degree centrality | 6.66E-03 | Betweenness centrality | 2.63E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.50E-01 | Radiality | 1.44E+01 | Clustering coefficient | 1.79E-01 |
| Neighborhood connectivity | 3.31E+01 | Topological coefficient | 4.65E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J Med Virol. 2020 Apr 28. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (BLA) 125496. | |||||
| REF 3 | ClinicalTrials.gov (NCT02760407) Evaluation of the Effectiveness and Safety of Two Dosing Regimens of Olokizumab (OKZ), Compared to Placebo and Adalimumab, in Subjects With Rheumatoid Arthritis (RA) Who Are Taking Methotrexate But Have Active Disease (CREDO 2). U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT01689532) A Study of CNTO 136 (Sirukumab) Administered Subcutaneously in Japanese Patients With Active Rheumatoid Arthritis Unresponsive to Methotrexate or Sulfasalazine. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT04348500) Clazakizumab (Anti-IL- 6 Monoclonal) Compared to Placebo for COVID19 Disease. U.S. National Institutes of Health. | |||||
| REF 6 | Siltuximab. In: Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US); 2006. 2018 Dec 3. | |||||
| REF 7 | Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS) J Autoimmun. 2020 Apr 10:102452. | |||||
| REF 8 | Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs. 2014 May-Jun;6(3):774-82. | |||||
| REF 9 | ClinicalTrials.gov (NCT04343989) A Randomized Placebo-controlled Safety and Dose-finding Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection. | |||||
| REF 10 | Sirukumab: A Potential Treatment for Mood Disorders Adv Ther. 2017 Jan;34(1):78-90. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

