Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02YIZ
|
||||
| Former ID |
DAP000111
|
||||
| Drug Name |
Erythromycin
|
||||
| Synonyms |
Abboticin; Abomacetin; Acneryne; Acnesol; Aknemycin; Aknin; AustriaS; Benzamycin; Derimer; Deripil; Dotycin; Dumotrycin; ERY; ERYC; Emgel; Emuvin; Emycin; Endoeritrin; Erecin; Erisone; Eritomicina; Eritrocina; Eritromicina; Ermycin; Eros; Eryacne; Eryacnen; Erycen; Erycette; Erycin; Erycinum; Eryderm; Erydermer; Erygel; Eryhexal; Erymax; Erymed; Erysafe; Erytab; Erythro; Erythroderm; Erythrogran; Erythroguent; Erythromid; Erythromycine; Erythromycinum; Erytop; Erytrociclin; Ilocaps; Iloticina; Ilotycin; Inderm; IndermRetcin; Latotryd; Lederpax; Mephamycin; Mercina; Oftamolets; Paediathrocin; Pantoderm; Pantodrin; Pantomicina; Pharyngocin; Primacine; Propiocine; Proterytrin; Retcin; Robimycin; Romycin; Sansac; Staticin; Stiemicyn; Stiemycin; Tiloryth; Tiprocin; Torlamicina; Wemid; Akne Cordes Losung; Aknederm Ery Gel; Benzamycin Pak; ERYTHROMYCIN STEARATE; Eryc Sprinkles; Erythromycin A; Erythromycin Lactate; Erythromycin Ointment; Erythromycin base; Erythromycin intravenous; Erythromycin sodium lauryl sulfate; Inderm Gel; Oftalmolosa Cusi Eritromicina; Skid Gel E; Theramycin Z; Udima Ery Gel; E0751; Eryc 125; Erythromast 36; Ak-Mycin; Akne-Mycin; Del-Mycin; E-Base; E-Glades; E-Mycin; E-Solve 2; ERYC (base); Emu-V; Emu-Ve; Erimycin-T; Eritromicina [INN-Spanish]; Ery-B; Ery-Diolan; Ery-Sol; Ery-Tab; Ery-maxin; Eryc (TN); Eryc-125; Eryc-250; Erygel (TN); Erythra-Derm; Erythro-Statin; Erythro-Teva; Erythromycin & VRC3375; Erythromycine [INN-French]; Erythromycinum [INN-Latin]; Ilosone (TN); Ilosone (estolate); Ilotycin T.S; Kesso-Mycin; N-Methylerythromycin A; PCE Dispertab (base); Pce (TN); R-P Mycin; Sans-acne; Staticin (TN); T-Stat; Taimoxin-F; A/T/S; Akne-mycin (TN); C-Solve-2; E-Base (base); E-Mycin (base); Ery-Tab (base); Erythromycin [INN:BAN:JAN]; Ilotycin T.S.; T-stat (TN); E-mycin, Erycin, Robimysin; Erythromycin (JP15/USP/INN); Erythromycin, compd. with monododecyl sulfate, sodium salt; Sulfuric acid, monododecyl ester, sodium salt, compd. with erythromycin; Adecane-2,10-dione (non-preferred name); Erythromycin A, T-Stat, Pantomicina, HSDB 3074, Erytab, DRG-0279; Ery
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Eli Lilly
|
||||
| Structure |
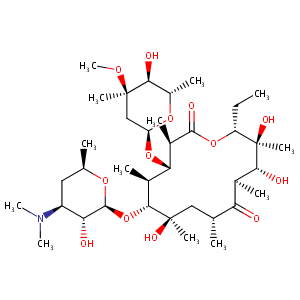
|
Download2D MOL |
|||
| Formula |
C37H67NO13
|
||||
| InChI |
InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
|
||||
| InChIKey |
ULGZDMOVFRHVEP-RWJQBGPGSA-N
|
||||
| CAS Number |
CAS 114-07-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
5020, 602912, 823926, 855292, 7847208, 7887354, 7979183, 8149330, 8159405, 10321305, 11335469, 11335572, 11335877, 11360708, 11360811, 11361116, 11362969, 11363553, 11365531, 11366115, 11368093, 11368677, 11374314, 11376255, 11376839, 11461680, 11461783, 11462088, 11484514, 11484962, 11488575, 11489079, 11492353, 11493929, 11494473, 12012581, 14720219, 14840178, 14864494, 25622201, 26611731, 26681121, 29280771, 46508487, 47365088, 47365089, 47662182, 47810649, 47885308, 47959639
|
||||
| ChEBI ID |
ChEBI:42355
|
||||
| SuperDrug ATC ID |
D10AF02; J01FA01; S01AA17
|
||||
| SuperDrug CAS ID |
cas=000114078
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | 50S ribosomal subunit | Target Info | Binder | [535286], [535445], [537356] | |
| References | |||||
| Ref 536773 | How many modes of action should an antibiotic have? Curr Opin Pharmacol. 2008 Oct;8(5):564-73. Epub 2008 Jul 30. | ||||
| Ref 538928 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1456). | ||||
| Ref 535286 | Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001 Oct 25;413(6858):814-21. | ||||
| Ref 535445 | Inhibition of 50S ribosomal subunit assembly in Haemophilus influenzae cells by azithromycin and erythromycin. Curr Microbiol. 2002 Jun;44(6):418-24. | ||||
| Ref 537356 | A synthetic alanyl-initiator tRNA with initiator tRNA properties as determined by fluorescence measurements: comparison to a synthetic alanyl-elongator tRNA. Nucleic Acids Res. 1991 Oct 25;19(20):5749-54. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

