Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08UQF
|
||||
| Former ID |
DNC000215
|
||||
| Drug Name |
Anthracycline
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Phase 2 | [526964] | ||
| Structure |
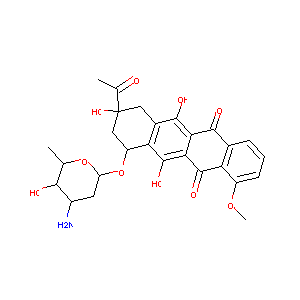
|
Download2D MOL |
|||
| Formula |
C27H29NO10
|
||||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C<br />=CC=C5OC)O)(C(=O)C)O)N)O
|
||||
| InChI |
1S/C27H29NO10/c1-10-22(30)14(28)7-17(37-10)38-16-9-27(35,11(2)29)8-13-19(16)26(34)21-20(24(13)32)23(31)12-5-4-6-15(36-3)18(12)25(21)33/h4-6,10,14,16-17,22,30,32,34-35H,7-9,28H2,1-3H3/t10-,14-,16-,17-,22+,27-/m0/s1
|
||||
| InChIKey |
STQGQHZAVUOBTE-VGBVRHCVSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
5016, 596008, 866161, 7887073, 7979028, 8171488, 11114094, 14763056, 15481415, 24769892, 26697306, 26701813, 26704223, 26715132, 26718351, 26718779, 29215022, 34672588, 46508433, 47440436, 47736668, 48110630, 48415843, 49699346, 49855159, 50070917, 50104250, 53788227, 56465013, 57288764, 57310919, 74382025, 74462219, 85788867, 92307396, 92308820, 96024474, 103477782, 104066339, 104307942, 117393873, 124766118, 124886869, 124886870, 124886871, 125299287, 126663226, 127301039, 127301040, 127301041
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Toposisomerase-1 | Target Info | Modulator | ||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| PANTHER Pathway | DNA replication | ||||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | ||||
| WikiPathways | Integrated Pancreatic Cancer Pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

