Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V3YT
|
||||
| Former ID |
DNC011192
|
||||
| Drug Name |
BOCEPREVIR
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
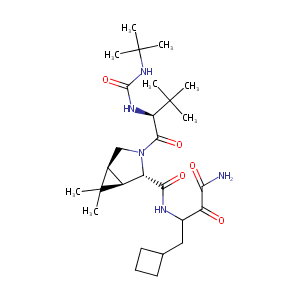
|
Download2D MOL |
|||
| Formula |
C27H45N5O5
|
||||
| InChI |
InChI=1S/C27H45N5O5/c1-25(2,3)20(30-24(37)31-26(4,5)6)23(36)32-13-15-17(27(15,7)8)18(32)22(35)29-16(19(33)21(28)34)12-14-10-9-11-14/h14-18,20H,9-13H2,1-8H3,(H2,28,34)(H,29,35)(H2,30,31,37)/t15-,16?,17-,18-,20+/m0/s1
|
||||
| InChIKey |
LHHCSNFAOIFYRV-DOVBMPENSA-N
|
||||
| CAS Number |
CAS 394730-60-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
15333889, 22433429, 22693607, 41390184, 46512784, 57374555, 57414843, 79603721, 92309409, 96025559, 103500897, 109692987, 134348397, 135216213, 137186019, 137251857, 139040316, 152258143, 160645839, 160646982, 162256796, 163667336, 164837112, 175427026, 175427127, 176245990, 180371765, 198992422, 223366205, 223669397, 226940314, 245972903, 249582863, 251970943, 252076908
|
||||
| SuperDrug ATC ID |
J05AE12
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Hepatitis C virus serine protease NS3/4A | Target Info | Modulator | [531783] | |
| References | |||||
| Ref 531783 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | ||||
| Ref 542802 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7876). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

