Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00HGB
|
||||
| Former ID |
DAP000972
|
||||
| Drug Name |
Niflumic Acid
|
||||
| Synonyms |
Actol; Donalgin; Flunir; Forenol; Landruma; NFL; Niflactol; Niflam; Niflugel; Niflumate; Nifluril; Acide niflumique; Acide niflumique [French]; Acido niflumico; Acido niflumico [Italian]; Acidum niflumicum; Nifluminic acid; UPSA Conseil Brand of Niflumic Acid; Upsamedica Brand of Niflumic Acid; N 0630; SC 1332; UP 83; UPSA Brand 1 of Niflumic Acid; UPSA Brand 2 of Niflumic Acid; Acid, Niflumic; Acide niflumique [INN-French]; Acido niflumico [INN-Spanish]; Acidum niflumicum [INN-Latin]; Niflugel (TN); Niflumic acid (INN); Niflumic acid [INN:DCF]; Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino; Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino [French]; 2-(3-(Trifluoromethyl)-phenyl)aminonicotinic acid; 2-(3-(Trifluoromethyl)anilino)nicotinic acid; 2-(3-Trifluoromethyl-phenylamino)-nicotinic acid; 2-(3-Trifluoromethylanilino)nicotinic Acid; 2-(3-[Trifluoromethyl]anilino)nicotinic acid; 2-(A,A,A-Trifluoro-m-toluidino)nicotinic acid; 2-(alpha,alpha,alpha-Trifluoro-m-toluidino)nicotinic acid; 2-[(3-TRIFLUOROMETHYL)PHENYL]AMINO-3-PYRIDINE-CARBOXYLIC ACID; 2-[(3-Trifluoromethyl)phenyl]amino]-3-pyridinecarboxylic Acid; 2-[(3-Trifluoromethylphenyl)amino]nicotinic Acid; 2-[3-(Trifluoromethyl)anilino]nicotinic acid; 2-[3-(trifluoromethyl)anilino]pyridine-3-carboxylic acid; 2-[alpha,alpha,alpha-trifluoro-m-toluidino]-nicotinic acid; 2-{[3-(TRIFLUOROMETHYL)PHENYL]AMINO}NICOTINIC ACID; 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid; 39690A
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiinflammatory Agents
|
||||
| Structure |
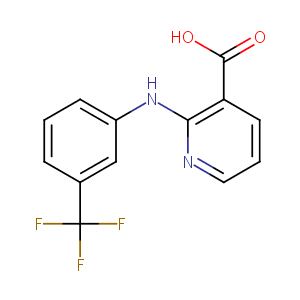
|
Download2D MOL |
|||
| Formula |
C13H9F3N2O2
|
||||
| InChI |
InChI=1S/C13H9F3N2O2/c14-13(15,16)8-3-1-4-9(7-8)18-11-10(12(19)20)5-2-6-17-11/h1-7H,(H,17,18)(H,19,20)
|
||||
| InChIKey |
JZFPYUNJRRFVQU-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 4394-00-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
586287, 832913, 855922, 3154432, 7403766, 7889362, 7980125, 8149792, 8152765, 10321830, 10522919, 10589648, 11111518, 11111519, 11119995, 11120483, 11120971, 11121458, 11121938, 11147078, 11335452, 11360691, 11362527, 11364247, 11365089, 11366809, 11367651, 11369371, 11370325, 11370326, 11372668, 11373252, 11373644, 11375813, 11377533, 11461663, 11466283, 11467403, 11484664, 11486025, 11488610, 11491300, 11491858, 11495167, 14720380, 14750946, 17405406, 24278582, 26612255, 26679975
|
||||
| SuperDrug ATC ID |
M01AX02; M02AA17
|
||||
| SuperDrug CAS ID |
cas=004394007
|
||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin G/H synthase 2 | Target Info | Inhibitor | [536416] | |
| KEGG Pathway | Arachidonic acid metabolism | ||||
| Metabolic pathways | |||||
| NF-kappa B signaling pathway | |||||
| VEGF signaling pathway | |||||
| TNF signaling pathway | |||||
| Retrograde endocannabinoid signaling | |||||
| Serotonergic synapse | |||||
| Ovarian steroidogenesis | |||||
| Oxytocin signaling pathway | |||||
| Regulation of lipolysis in adipocytes | |||||
| Leishmaniasis | |||||
| Pathways in cancer | |||||
| Chemical carcinogenesis | |||||
| MicroRNAs in cancer | |||||
| Small cell lung cancer | |||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| References | |||||
| Ref 539567 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2439). | ||||
| Ref 550701 | Drug information of Niflumic Acid, 2008. eduDrugs. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

