Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00YZD
|
||||
| Former ID |
DNCL002987
|
||||
| Drug Name |
Dolutegravir
|
||||
| Synonyms |
S/GSK1349572; DTG
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
GlaxoSmithKline
|
||||
| Structure |
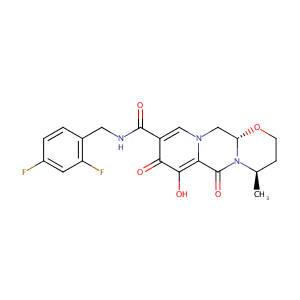
|
Download2D MOL |
|||
| Formula |
C20H19F2N3O5
|
||||
| InChI |
InChI=1S/C20H19F2N3O5/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28)/t10-,15+/m1/s1
|
||||
| InChIKey |
RHWKPHLQXYSBKR-BMIGLBTASA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
96100998, 123055423, 124361075, 124361082, 124361088, 135268073, 135273443, 135626786, 136340316, 136349541, 136367672, 136920429, 144115916, 152258477, 160647312, 160693271, 162011364, 162038052, 162202774, 163677014, 164043525, 164193963, 164347381, 170484068, 170484069, 170484070, 170503405, 174007466, 174525979, 175427161, 178103937, 184812380, 189622844, 198935766, 198993556, 223382143, 223705174, 226460906, 242590891, 248390482, 250184664, 252110159, 252224388, 252451605
|
||||
| SuperDrug ATC ID |
J05AX12
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | HIV integrase | Target Info | Modulator | [532651] | |
| References | |||||
| Ref 532651 | 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85-9. | ||||
| Ref 542387 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7365). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

